| 1 |
ClinicalTrials.gov (NCT00885300) Cloxacillin as Prevention of Double Lumen Infection in Hemodialysis Patients
|
| 2 |
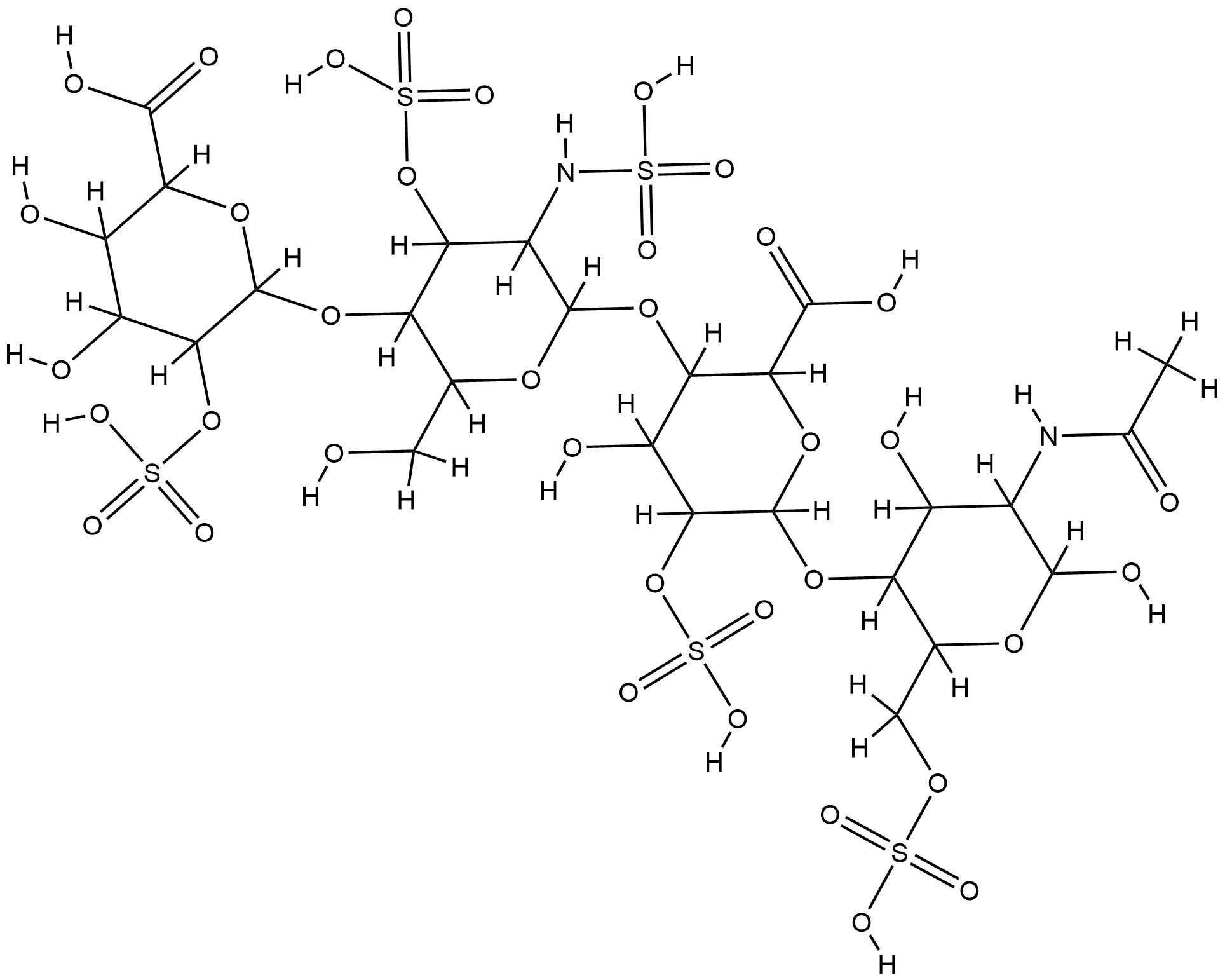
Heparin FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 090809.
|
| 4 |
Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020 Dec;9(1):727-732.
|
| 5 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 061454.
|
| 6 |
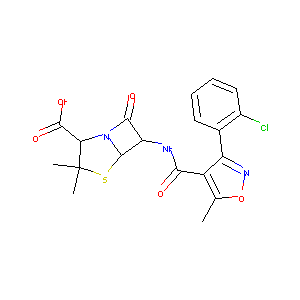
Cloxacillin FDA Label
|
| 7 |
Development of a receptor-based microplate assay for the detection of beta-lactam antibiotics in different food matrices. Anal Chim Acta. 2007 Mar 14;586(1-2):296-303.
|
| 8 |
Potential cholestatic activity of various therapeutic agents assessed by bile canalicular membrane vesicles isolated from rats and humans. Drug Metab Pharmacokinet. 2003;18(1):16-22.
|
| 9 |
Pro-inflammatory cytokines enhance dilatation of bile canaliculi caused by cholestatic antibiotics. Toxicol In Vitro. 2019 Aug;58:51-59. doi: 10.1016/j.tiv.2019.03.015. Epub 2019 Mar 12.
|
|
|
|
|
|
|