Details of the Drug
General Information of Drug (ID: DM4ZP3W)
| Drug Name |
Heparin
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
enoxaparin; Ardeparin; Bemiparin; SEMULOPARIN; Lovenox; LMWH; Nadroparin; Nadroparine; Reviparin; Clivarin; Sublingula; Sandoparin; Parvoparin; Parnaparin; Novoheparin; Adomiparin; Tinzaparin; Multiparin; Hepathrom; Heparina; Triofiban; Pularin; Liquemin; Heparinum; Subeparin; PK-10169; Pabyrin; Octaparin; Liquaemin; Heparine; Vetren; Fluxum; Hed-heparin; Depo-Heparin; Lipo-hepin; Fragmin A; Fragmin B; Vitrum AB; Heparin [BAN]; Eparina [DCIT]; CY 216; Adomiparin [USAN]; FR 860; Heparin CY 216; Heparine [INN-French]
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
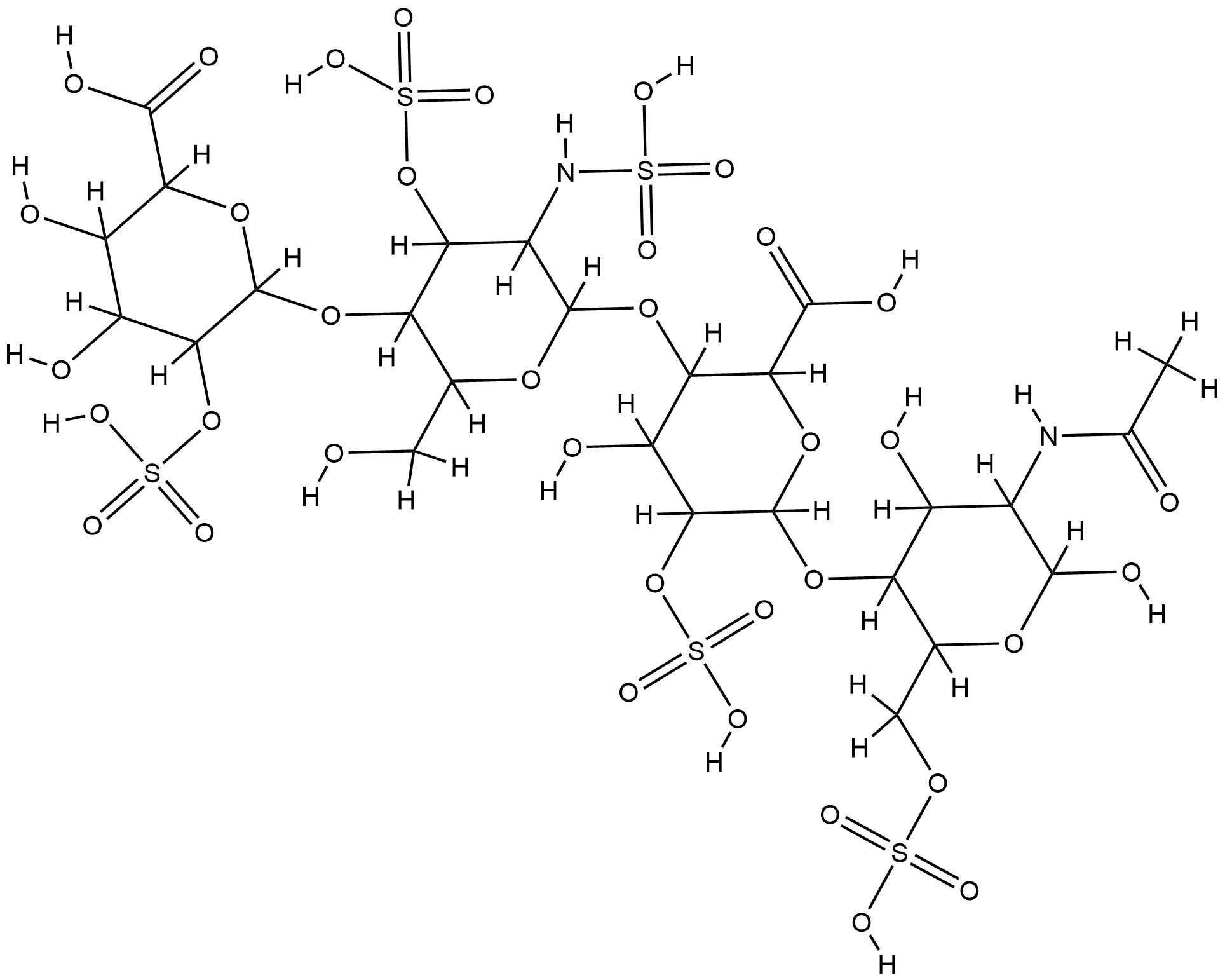 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL is unavailable | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
