| 1 |
ClinicalTrials.gov (NCT02655614) A Study of GDC-0134 to Determine Initial Safety, Tolerability, and Pharmacokinetic Parameters in Participants With Amyotrophic Lateral Sclerosis
|
| 2 |
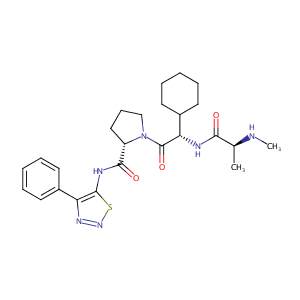
Discovery of a potent small-molecule antagonist of inhibitor of apoptosis (IAP) proteins and clinical candidate for the treatment of cancer (GDC-0152). J Med Chem. 2012 May 10;55(9):4101-13.
|
| 3 |
Small molecules, big targets: drug discovery faces the protein-protein interaction challenge.Nat Rev Drug Discov. 2016 Aug;15(8):533-50.
|
| 4 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7290).
|
| 5 |
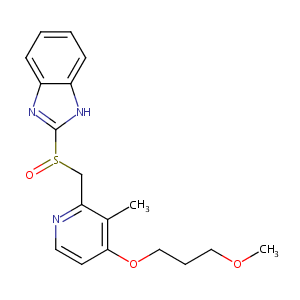
Rabeprazole FDA Label
|
| 6 |
Review article: rabeprazole's tolerability profile in clinical trials. Aliment Pharmacol Ther. 1999 Oct;13 Suppl 5:17-23.
|
| 7 |
Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009 Jan;67(1):44-9.
|
| 8 |
Effects of clarithromycin and verapamil on rabeprazole pharmacokinetics between CYP2C19 genotypes. Eur J Clin Pharmacol. 2006 Aug;62(8):597-603.
|
|
|
|
|
|
|