| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
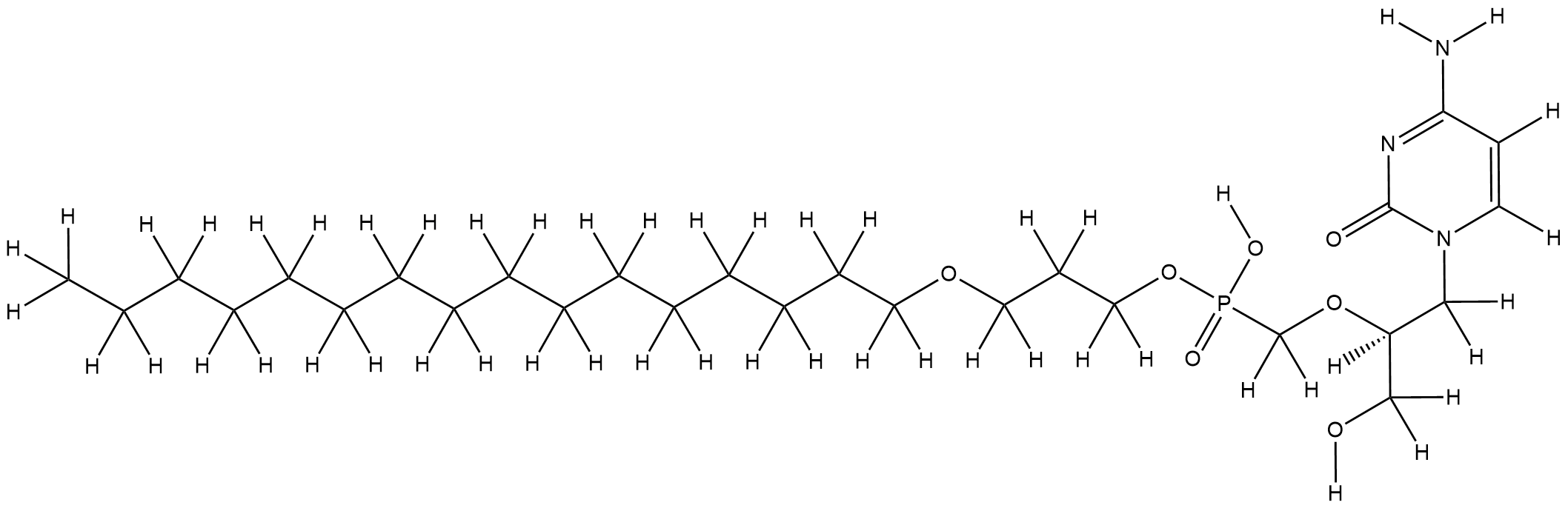
A rational roadmap for SARS-CoV-2/COVID-19 pharmacotherapeutic research and development: IUPHAR Review 29. Br J Pharmacol. 2020 May 1;10.1111/bph.15094.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7303).
|
| 4 |
Aromatase inhibitors for male infertility. J Urol. 2002 Feb;167(2 Pt 1):624-9.
|
| 5 |
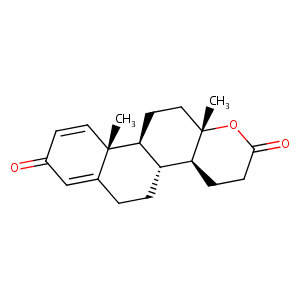
Aromatase inhibitors in precocious puberty: rationale and experience to date. Treat Endocrinol. 2004;3(3):141-51. doi: 10.2165/00024677-200403030-00002.
|
|
|
|
|
|
|