Details of the Drug
General Information of Drug (ID: DMVY4GN)
| Drug Name |
Testolactone
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fludestrin; Teolit; Teslac; Teslak; Testolacton; Testolactona; Testolactonum; Testolattone; Bristol Myers SquibbBrand of Testolactone; Testolattone [DCIT]; SQ 9538; Bristol-Myers Squibb Brand of Testolactone; DELTA1-Dehydrotestolactone; DELTA1-Dehydrotestololactone; DELTA1-Testololactone; SQ-9538; TESLAC (TN);Teslac (TN); Testolactona [INN-Spanish]; Testolactone [USAN:INN]; Testolactonum [INN-Latin]; Delta(1)-Dehydrotestolactone; Delta(1)-Testolactone; Delta(1)-Testololactone; Delta(1)-testololactone; Delta-1-testololactone; Testolactone (USP/INN); D-Homo-17A-oxaandrosta-1,4-diene-3,17-dione; D-homo-17a-oxaandrosta-1,4-diene-3,17-dione; (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-3,4,4a,5,6,10a,10b,11,12,12a-decahydro-2H-naphtho[2,1-f]chromene-2,8(4bH)-dione; (4aS,4bR,10aR,10bS,12aS)-10a,12a-dimethyl-4,4a,4b,5,6,10b,11,12-octahydro-3H-naphtho[2,1-f]chromene-2,8-dione; 1 Dehydrotestolactone; 1,2,3,4,4a,4b,7,9,10,10a-Decahydro-2-hydroxy-2,4b-dimethyl-7-oxo-1-phenanthrenepropionic acid delta-lactone; 1,2-Dehydrotestololactone; 1,2-Didehydrotestololactone; 1,2-didehydrotestololactone; 1-Dehydrotestolactone; 1-Dehydrotestololactone; 1-dehydrotestololactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, .delta.-lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, delta-lactone (8CI); 13,17-Secoandrosta-1,4-dien-17-oic acid, 13-hydroxy-3-oxo-, lactone; 13,17-Secoandrosta-1,4-dien-17-oic acid, 13.alpha.-hydroxy-3-oxo-, .delta.-lactone; 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid .delta.-lactone; 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone; 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid delta-lactone; 17a-Oxa-D-homoandrosta-1,4-diene-3,17-dione; 17alpha-Oxo-D-homo-1,4-androstadiene-3,17-dione; 2H-Phenanthro[2,1-b]pyran-2,8(4bH)-dione, 3,4,4a,5,6,10a,10b,11,12,12a-decahydro-10a,12a-dimethyl-, lactone; 3-oxo-13,17-secoandrosta-1,4-dieno-17,13alpha-lactone
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Anticancer Agents
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
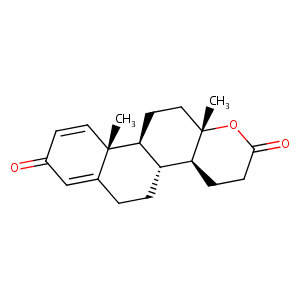 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 300.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Testolactone (Comorbidity)
|
|||||||||||||||||||||||||||||||||||
References
