| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076923.
|
| 3 |
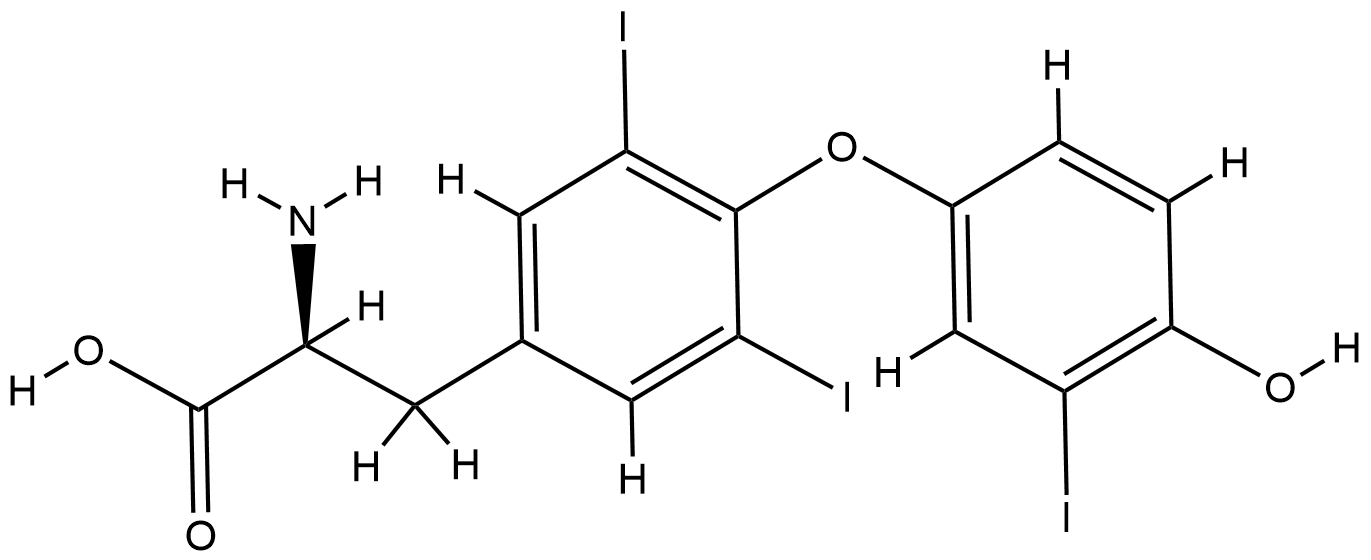
ClinicalTrials.gov (NCT04348513) Triiodothyronine for the Treatment of Critically Ill Patients With COVID-19 Infection. U.S. National Institutes of Health.
|
| 4 |
Approved antiretroviral drugs. Antiretroviral Drugs. Company report of AVERT. 2009.
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 6 |
Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007.
|
| 7 |
Induction of multiple drug transporters by efavirenz. J Pharmacol Sci. 2009 Feb;109(2):242-50.
|
| 8 |
Induction of CYP3A4 by efavirenz in primary human hepatocytes: comparison with rifampin and phenobarbital. J Clin Pharmacol. 2004 Nov;44(11):1273-81.
|
| 9 |
CYP2B6 genotype-dependent inhibition of CYP1A2 and induction of CYP2A6 by the antiretroviral drug efavirenz in healthy volunteers. Clin Transl Sci. 2019 Nov;12(6):657-666.
|
| 10 |
Evaluation of CYP2B6 induction and prediction of clinical drug-drug interactions: considerations from the IQ consortium induction working group-an industry perspective. Drug Metab Dispos. 2016 Oct;44(10):1720-30.
|
| 11 |
Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos. 2006 Oct;34(10):1742-8.
|
| 12 |
Inhibition of human cytochrome P450 isoforms by nonnucleoside reverse transcriptase inhibitors. J Clin Pharmacol. 2001 Jan;41(1):85-91.
|
| 13 |
Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43.
|
| 14 |
Lon protease: a novel mitochondrial matrix protein in the interconnection between drug-induced mitochondrial dysfunction and endoplasmic reticulum stress. Br J Pharmacol. 2017 Dec;174(23):4409-4429. doi: 10.1111/bph.14045. Epub 2017 Nov 7.
|
| 15 |
Efavirenz and 8-hydroxyefavirenz induce cell death via a JNK- and BimEL-dependent mechanism in primary human hepatocytes. Toxicol Appl Pharmacol. 2011 Dec 1;257(2):227-34. doi: 10.1016/j.taap.2011.09.008. Epub 2011 Sep 19.
|
| 16 |
Reverse transcriptase inhibitors down-regulate cell proliferation in vitro and in vivo and restore thyrotropin signaling and iodine uptake in human thyroid anaplastic carcinoma. J Clin Endocrinol Metab. 2005 Oct;90(10):5663-71. doi: 10.1210/jc.2005-0367. Epub 2005 Jul 19.
|
| 17 |
Binding of anti-HIV drugs to human serum albumin. IUBMB Life. 2004 Oct;56(10):609-14. doi: 10.1080/15216540400016286.
|
| 18 |
Effects of nevirapine and efavirenz on human adipocyte differentiation, gene expression, and release of adipokines and cytokines. Antiviral Res. 2011 Aug;91(2):112-9. doi: 10.1016/j.antiviral.2011.04.018. Epub 2011 May 17.
|
|
|
|
|
|
|