Details of the Drug
General Information of Drug (ID: DM14NKM)
| Drug Name |
Triiodothyronine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
liothyronine; triiodothyronine; 3,3',5-Triiodo-L-thyronine; 6893-02-3; Liothyronin; Tresitope; 3,5,3'-triiodothyronine; L-Liothyronine; O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine; 3,5,3'-Triiodo-L-thyronine; triothyrone; Liothyroninum; Lyothyronine; Liotironina; T3; L-T3; 3,3',5-Triiodothyronine; Triiodo-L-thyronine; 3,5,3'TRIIODOTHYRONINE; L-3,5,3'-Triiodothyronine; T3 (amino acid); T3 (Hormone); L-3,3',5-TriioDOThyronine; Liothyronine [INN:BAN]; Li; Liothyronine
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
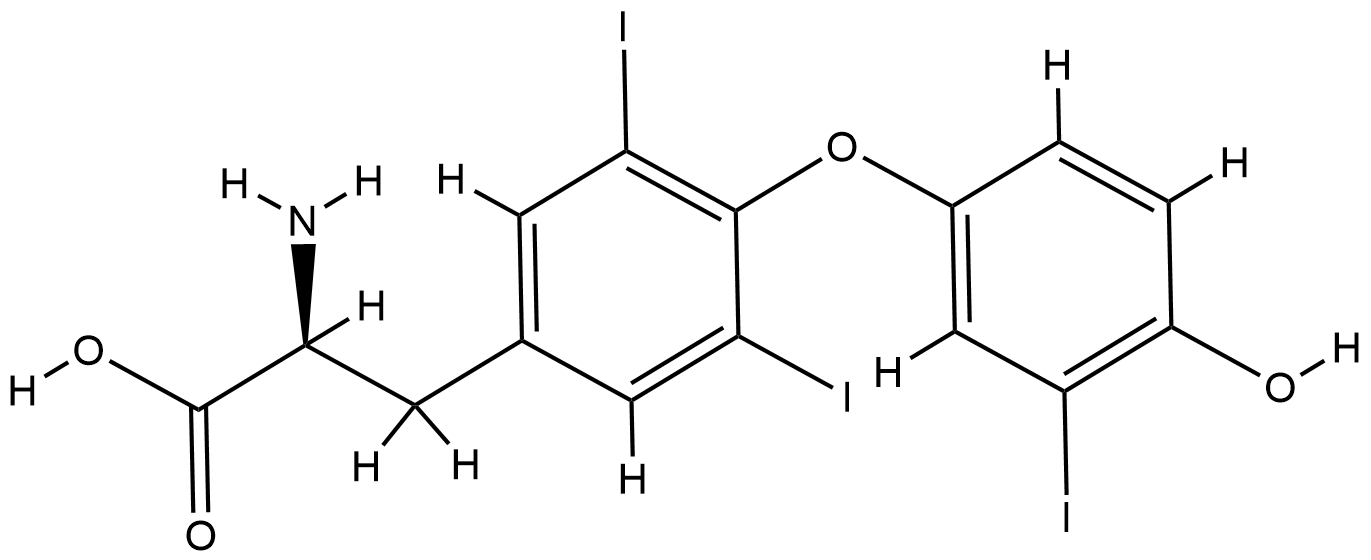 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
