| 1 |
ClinicalTrials.gov (NCT03320031) A Study of Adding Linagliptin to Control Glycemic Variability and HbA1c in Peritoneal Dialysis Patients With Type 2 Diabetes(PDPD) With Premixed Insulin Therapy
|
| 2 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 3 |
Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006 Jul;12(7):RA130-47. Epub 2006 Jun 28.
|
| 4 |
Multicenter, Randomized Trial of a Bionic Pancreas in Type 1 Diabetes. N Engl J Med. 2022 Sep 29;387(13):1161-1172.
|
| 5 |
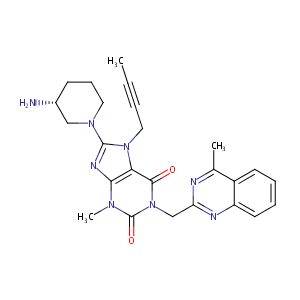
Linagliptin FDA Label
|
| 6 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (NDA) 208026
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6318).
|
| 8 |
ClinicalTrials.gov (NCT04371978) Efficacy and Safety of Dipeptidyl Peptidase-4 Inhibitors in Diabetic Patients With Established COVID-19. U.S. National Institutes of Health.
|
| 9 |
Hope for insulin mimetic oral antidiabetic drugs. Eur J Endocrinol. 1999 Dec;141(6):561-2.
|
| 10 |
Boehringer Ingelheim. Product Development Pipeline. June 2 2009.
|
| 11 |
DPP4 inhibition: preventing SARS-CoV-2 infection and/or progression of COVID-19 Diabetes Metab Res Rev. 2020 Apr 26.
|
| 12 |
Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition.
|
|
|
|
|
|
|