| 1 |
ClinicalTrials.gov (NCT01885689) Clofarabine and Melphalan Before Donor Stem Cell Transplant in Treating Patients With Myelodysplasia, Acute Leukemia in Remission, or Chronic Myelomonocytic Leukemia
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6802).
|
| 3 |
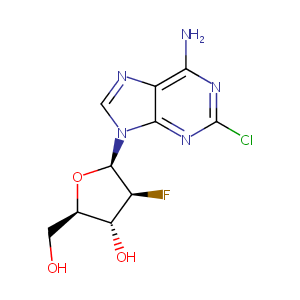
Clofarabine FDA Label
|
| 4 |
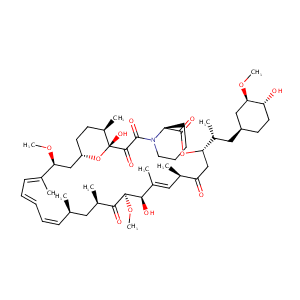
Sirolimus FDA Label
|
| 5 |
FDA Approved Drug Products from FDA Official Website. 2019. Application Number: (ANDA) 201578.
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6031).
|
| 8 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 9 |
ClinicalTrials.gov (NCT04341675) Sirolimus Treatment in Hospitalized Patients With COVID-19 Pneumonia. U.S. National Institutes of Health.
|
| 10 |
Clofarabine: past, present, and future. Leuk Lymphoma. 2007 Oct;48(10):1922-30.
|
| 11 |
Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008 Sep;7(9):3092-102.
|
| 12 |
Cytarabine-resistant leukemia cells are moderately sensitive to clofarabine in vitro. Anticancer Res. 2014 Apr;34(4):1657-62.
|
| 13 |
FDA label of Belinostat. The 2020 official website of the U.S. Food and Drug Administration.
|
| 14 |
Identification of environmental chemicals that activate p53 signaling after in vitro metabolic activation. Arch Toxicol. 2022 Jul;96(7):1975-1987. doi: 10.1007/s00204-022-03291-5. Epub 2022 Apr 18.
|
| 15 |
Knockdown of Bcl-xL enhances growth-inhibiting and apoptosis-inducing effects of resveratrol and clofarabine in malignant mesothelioma H-2452 cells. J Korean Med Sci. 2014 Nov;29(11):1464-72. doi: 10.3346/jkms.2014.29.11.1464. Epub 2014 Nov 4.
|
| 16 |
Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51.
|
| 17 |
The genomic landscape of nasopharyngeal carcinoma.Nat Genet. 2014 Aug;46(8):866-71.
|
| 18 |
Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020 Mar 16;6:14.
|
| 19 |
Pharmacogenetics of tacrolimus and sirolimus in renal transplant patients: from retrospective analyses to prospective studies. Transplant Proc. 2007 Sep;39(7):2142-4.
|
| 20 |
Pharmacokinetic and pharmacodynamic interactions between the immunosuppressant sirolimus and the lipid-lowering drug ezetimibe in healthy volunteers. Clin Pharmacol Ther. 2010 Jun;87(6):663-7.
|
| 21 |
Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev. 2002 Feb-May;34(1-2):83-448.
|
| 22 |
Drug Interactions Flockhart Table
|
| 23 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
|
|
|
|
|
|