| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 3 |
ClinicalTrials.gov (NCT01836458) A Study to Find the Minimum Inhibitory Concentration of KAE609 in Adult Male Patients With P. Falciparum Monoinfection. U.S. National Institutes of Health.
|
| 4 |
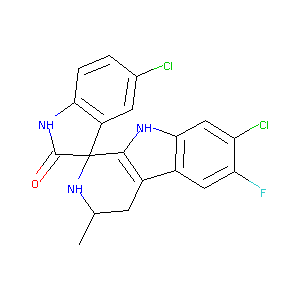
Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010 Sep 3;329(5996):1175-80.
|
|
|
|
|
|
|