Details of the Drug
General Information of Drug (ID: DMQHBSX)
| Drug Name |
NITD609
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Cipargamin; 1193314-23-6; NITD-609; NITD 609; UNII-Z7Q4FWA04P; (1'R,3'S)-5,7'-Dichloro-6'-fluoro-3'-methyl-2',3',4',9'-tetrahydrospiro[indoline-3,1'-pyrido[3,4-b]indol]-2-one; Z7Q4FWA04P; CHEMBL1082723; Spiro[3H-indole-3,1'-[1H]pyrido[3,4-b]indol]-2(1H)-one, 5,7'-dichloro-6'-fluoro-2',3',4',9'-tetrahydro-3'-methyl-, (1'R,3'S)-; Cipargamin [INN]; 1252008-89-1; GTPL9721; SCHEMBL1306342; DTXSID70152424; MolPort-039-337-269; ZINC49037032; BDBM50318666; AKOS027253851; SB16518; DB12306; CS-7486; NCGC00263785-01
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
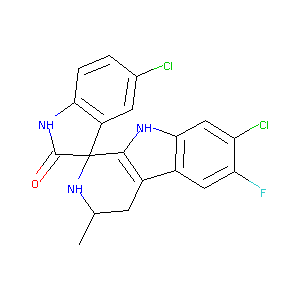 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 390.2 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
References
