| 1 |
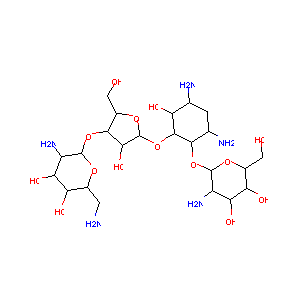
ClinicalTrials.gov (NCT03129646) Miltefosine/Paromomycin Phase III Trial for Treatment of Primary Visceral Leishmaniasis (VL) Patients in Eastern Africa
|
| 2 |
Paromomycin FDA Label
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 064171.
|
| 4 |
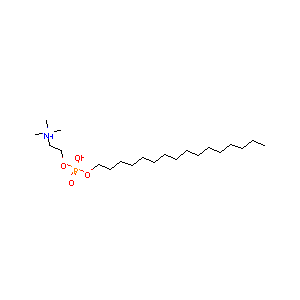
Miltefosine FDA Label
|
| 5 |
2014 FDA drug approvals. Nat Rev Drug Discov. 2015 Feb;14(2):77-81.
|
| 6 |
Novel antifungal agents, targets or therapeutic strategies for the treatment of invasive fungal diseases: a review of the literature (2005-2009). Rev Iberoam Micol. 2009 Mar 31;26(1):15-22.
|
| 7 |
Aminoglycoside association pathways with the 30S ribosomal subunit. J Phys Chem B. 2009 May 21;113(20):7322-30.
|
| 8 |
Correction of ATM gene function by aminoglycoside-induced read-through of premature termination codons. Proc Natl Acad Sci U S A. 2004 Nov 2;101(44):15676-81. doi: 10.1073/pnas.0405155101. Epub 2004 Oct 21.
|
| 9 |
Development of miltefosine as an oral treatment for leishmaniasis. Trans R Soc Trop Med Hyg. 2006 Dec;100 Suppl 1:S17-20.
|
| 10 |
Dimethylsphingosine and miltefosine induce apoptosis in lung adenocarcinoma A549?cells in a synergistic manner. Chem Biol Interact. 2019 Sep 1;310:108731. doi: 10.1016/j.cbi.2019.108731. Epub 2019 Jun 29.
|
| 11 |
MDR1 causes resistance to the antitumour drug miltefosine. Br J Cancer. 2001 May 18;84(10):1405-11. doi: 10.1054/bjoc.2001.1776.
|
|
|
|
|
|
|