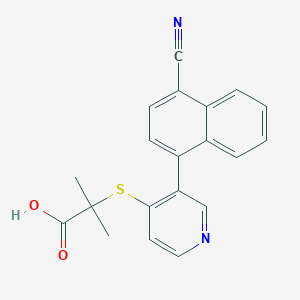
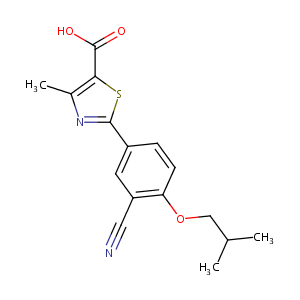
| 1 |
ClinicalTrials.gov (NCT03316131) A Study to Assess the Effect of Intensive Uric Acid (UA) Lowering Therapy With RDEA3170, Febuxostat, Dapagliflozin on Urinary Excretion of UA
|
| 2 |
ClinicalTrials.gov (NCT02078219) Phase II Dose Finding Study of RDEA3170 Versus Placebo in Japanese Patients With Gout or Asymptomatic Hyperuricemia. U.S. National Institutes of Health.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6817).
|
| 4 |
The pathophysiology of hyperuricaemia and its possible relationship to cardiovascular disease, morbidity and mortality. BMC Nephrol. 2013; 14: 164.
|
| 5 |
Febuxostat Increases Ventricular Arrhythmogenesis Through Calcium Handling Dysregulation in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol Sci. 2022 Sep 24;189(2):216-224. doi: 10.1093/toxsci/kfac073.
|
| 6 |
Clinical pipeline report, company report or official report of Takeda (2009).
|
| 7 |
In vitro drug-drug interaction studies with febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibition. Xenobiotica. 2008 May;38(5):496-510.
|
| 8 |
Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol. 2016 Sep;36(9):1120-8. doi: 10.1002/jat.3272. Epub 2015 Dec 7.
|
|
|
|
|
|
|