Details of the Drug
General Information of Drug (ID: DMDEXQ0)
| Drug Name |
Febuxostat
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Adenuric; TEI; Uloric; Febuxostat [USAN]; S1547; TMX 67; Tei 6720; TMX-67; Tei-6720; Uloric (TN); Febuxostat (JAN/USAN/INN); TMX-67, Adenuric, Uloric, Febuxostat; 111GE013; 2-(3-CYANO-4-ISOBUTOXY-PHENYL)-4-METHYL-5-THIAZOLE-CARBOXYLIC ACID; 2-(3-Cyano-4-(2-methylpropoxy)phenyl)-4-methylthiazole-5-carboxylic acid;2-(3-Cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylic acid; 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-1,3-thiazole-5-carboxylic acid
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
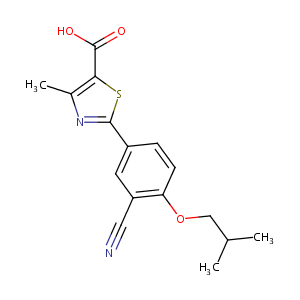 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 316.4 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.9 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 6 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Febuxostat (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6817). | ||||
|---|---|---|---|---|---|
| 2 | Hu M, Tomlinson B: Febuxostat in the management of hyperuricemia and chronic gout: a review. Ther Clin Risk Manag. 2008 Dec;4(6):1209-20. doi: 10.2147/tcrm.s3310. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | FDA Approved Products: Uloric (febuxostat) oral tablets | ||||
| 5 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 6 | Grabowski BA, Khosravan R, Vernillet L, Mulford DJ: Metabolism and excretion of [14C] febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase, in healthy male subjects. J Clin Pharmacol. 2011 Feb;51(2):189-201. doi: 10.1177/0091270010365549. Epub 2010 Mar 30. | ||||
| 7 | Clinical pipeline report, company report or official report of Takeda (2009). | ||||
| 8 | In vitro drug-drug interaction studies with febuxostat, a novel non-purine selective inhibitor of xanthine oxidase: plasma protein binding, identification of metabolic enzymes and cytochrome P450 inhibition. Xenobiotica. 2008 May;38(5):496-510. | ||||
| 9 | Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol. 2016 Sep;36(9):1120-8. doi: 10.1002/jat.3272. Epub 2015 Dec 7. | ||||
| 10 | Febuxostat Increases Ventricular Arrhythmogenesis Through Calcium Handling Dysregulation in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Toxicol Sci. 2022 Sep 24;189(2):216-224. doi: 10.1093/toxsci/kfac073. | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 12 | Product Information. Sirturo (bedaquiline). Janssen Pharmaceuticals, Titusville, NJ. | ||||
| 13 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 14 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 15 | Product Information. Adcetris (brentuximab vedotin). Seattle Genetics Inc, Bothell, WA. | ||||
| 16 | Product Information. Kynamro (mipomersen). Genzyme Corporation, Cambridge, MA. | ||||
| 17 | Canadian Pharmacists Association. | ||||
| 18 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 19 | Al-Nawakil C, Willems L, Mauprivez C, et.al "Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors." Leuk Lymphoma 55 (2014): 1670-4. [PMID: 24090500] | ||||
| 20 | Product Information. Zydelig (idelalisib). Gilead Sciences, Foster City, CA. | ||||
