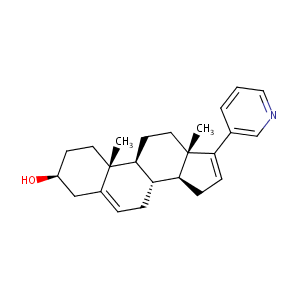
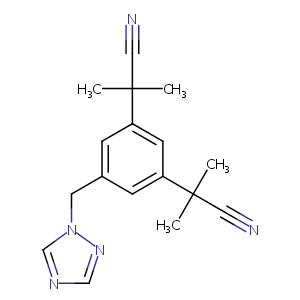
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6745).
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 5137).
|
| 4 |
Anastrozole FDA Label
|
| 5 |
2011 FDA drug approvals. Nat Rev Drug Discov. 2012 Feb 1;11(2):91-4.
|
| 6 |
A fission yeast-based test system for the determination of IC50 values of anti-prostate tumor drugs acting on CYP21. J Enzyme Inhib Med Chem. 2006 Oct;21(5):547-56.
|
| 7 |
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects. Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008.
|
| 8 |
Targeting CD46 for both adenocarcinoma and neuroendocrine prostate cancer. JCI Insight. 2018 Sep 6;3(17):e121497. doi: 10.1172/jci.insight.121497. eCollection 2018 Sep 6.
|
| 9 |
Targeting chromatin binding regulation of constitutively active AR variants to overcome prostate cancer resistance to endocrine-based therapies. Nucleic Acids Res. 2015 Jul 13;43(12):5880-97. doi: 10.1093/nar/gkv262. Epub 2015 Apr 23.
|
| 10 |
Effective aromatase inhibition by anastrozole in a patient with gonadotropin-independent precocious puberty in McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2002;15 Suppl 3:945-8.
|
| 11 |
Anastrozole Aromatase Inhibitor Plasma Drug Concentration Genome-Wide Association Study: Functional Epistatic Interaction Between SLC38A7 and ALPPL2. Clin Pharmacol Ther. 2019 Jan 16.
|
| 12 |
In vitro and in vivo oxidative metabolism and glucuronidation of anastrozole. Br J Clin Pharmacol. 2010 Dec;70(6):854-69.
|
| 13 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 14 |
Aromatase inhibitors: cellular and molecular effects. J Steroid Biochem Mol Biol. 2005 May;95(1-5):83-9. doi: 10.1016/j.jsbmb.2005.04.010.
|
| 15 |
Aromatase inhibition: translation into a successful therapeutic approach. Clin Cancer Res. 2005 Apr 15;11(8):2809-21. doi: 10.1158/1078-0432.CCR-04-2187.
|
| 16 |
Assessment of Five Pesticides as Endocrine-Disrupting Chemicals: Effects on Estrogen Receptors and Aromatase. Int J Environ Res Public Health. 2022 Feb 10;19(4):1959. doi: 10.3390/ijerph19041959.
|
| 17 |
Inhibition of estrogen receptor reduces connexin 43 expression in breast cancers. Toxicol Appl Pharmacol. 2018 Jan 1;338:182-190. doi: 10.1016/j.taap.2017.11.020. Epub 2017 Nov 24.
|
| 18 |
Biomarker changes during neoadjuvant anastrozole, tamoxifen, or the combination: influence of hormonal status and HER-2 in breast cancer--a study from the IMPACT trialists. J Clin Oncol. 2005 Apr 10;23(11):2477-92. doi: 10.1200/JCO.2005.07.559. Epub 2005 Mar 14.
|
| 19 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
|
|
|
|
|
|