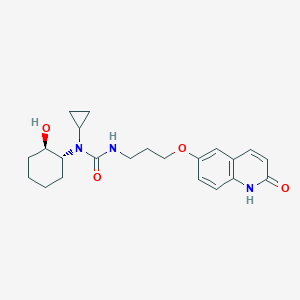
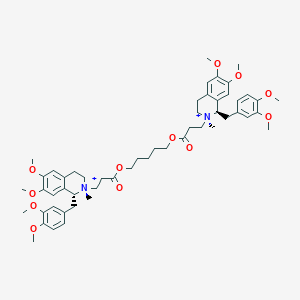
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
ClinicalTrials.gov (NCT00783081) Safety and Efficacy of K-134 for the Treatment of Intermittent Claudication. U.S. National Institutes of Health.
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
A phase II dose-ranging study of the phosphodiesterase inhibitor K-134 in patients with peripheral artery disease and claudication. J Vasc Surg. 2012 Feb;55(2):381-389.e1.
|
| 5 |
Bradycardia produced by pyridostigmine and physostigmine. Can J Anaesth. 1997 Dec;44(12):1286-92.
|
| 6 |
Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009 Jun;110(6):1244-52.
|
|
|
|
|
|
|