| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 6585).
|
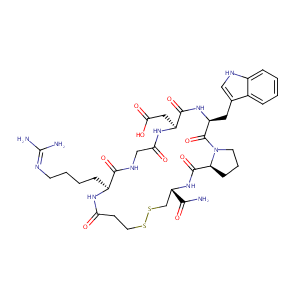
| 3 |
Eptifibatide FDA Label
|
| 4 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800022099)
|
| 5 |
Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. J Biol Chem. 1991 May 25;266(15):9359-62.
|
| 6 |
Eptifibatide in peripheral vascular interventions: results of the Integrilin Reduces Inflammation in Peripheral Vascular Interventions (INFLAME) trial. J Invasive Cardiol. 2006 Jan;18(1):6-12.
|
| 7 |
ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899.
|
| 8 |
Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br J Pharmacol. 2007 Jul;151(6):737-48.
|
| 9 |
Erythromycin interaction with risperidone or clomipramine in an adolescent. J Child Adolesc Psychopharmacol. 1996 Summer;6(2):133-8.
|
| 10 |
Role of human UGT2B10 in N-glucuronidation of tricyclic antidepressants, amitriptyline, imipramine, clomipramine, and trimipramine. Drug Metab Dispos. 2010 May;38(5):863-70.
|
| 11 |
The biotransformation of clomipramine in vitro, identification of the cytochrome P450s responsible for the separate metabolic pathways. J Pharmacol Exp Ther. 1996 Jun;277(3):1659-64.
|
| 12 |
Serum clomipramine and desmethylclomipramine levels in a CYP2C19 and CYP2D6 intermediate metabolizer. Pharmacogenomics. 2017 May;18(7):601-605.
|
|
|
|
|
|
|