| 1 |
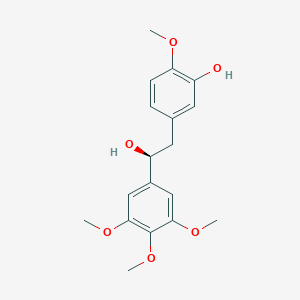
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 3 |
ClinicalTrials.gov (NCT00618319) An Open-Label, 18FDG-PET Pharmacodynamic Assessment of the Effect of BIIB021 in Subjects With Gastrointestinal Stromal Tumors (GIST). U.S. National Institutes of Health.
|
| 4 |
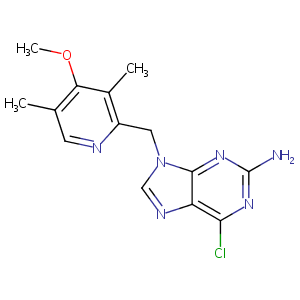
BIIB021, an orally available, fully synthetic small-molecule inhibitor of the heat shock protein Hsp90. Mol Cancer Ther. 2009 Apr;8(4):921-9.
|
|
|
|
|
|
|