Details of the Drug
General Information of Drug (ID: DM9R0ZL)
| Drug Name |
CA4P
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Fosbretabulin disodium; 168555-66-6; Combretastatin A4 disodium phosphate; CA4DP; Fosbretabulin (disodium); Zybrestat; UNII-702RHR475O; Combretastatin A4 Phosphate Disodium Salt; Fosbretabulin disodium [USAN]; 702RHR475O; Combretastatin A-4 phosphate; Fosbretabulin disodium (USAN); CHEMBL289351; Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium; AC1OCF9S; Fosbretabulin Disodium Salt; CA-4P; CA 4P; SCHEMBL321426; C18H19O8P.2Na; MolPort-006-823-175; ACT03122; 3559AH; s7204; AKOS027327946; BCP9000542; CS-1484; NSC-752293; Fosbr
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
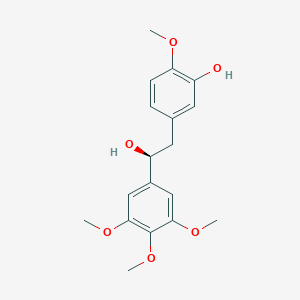
| Structure |
 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
