| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 3 |
FDA Approved Drug Products from FDA Official Website. 2009. Application Number: (ANDA) 076923.
|
| 4 |
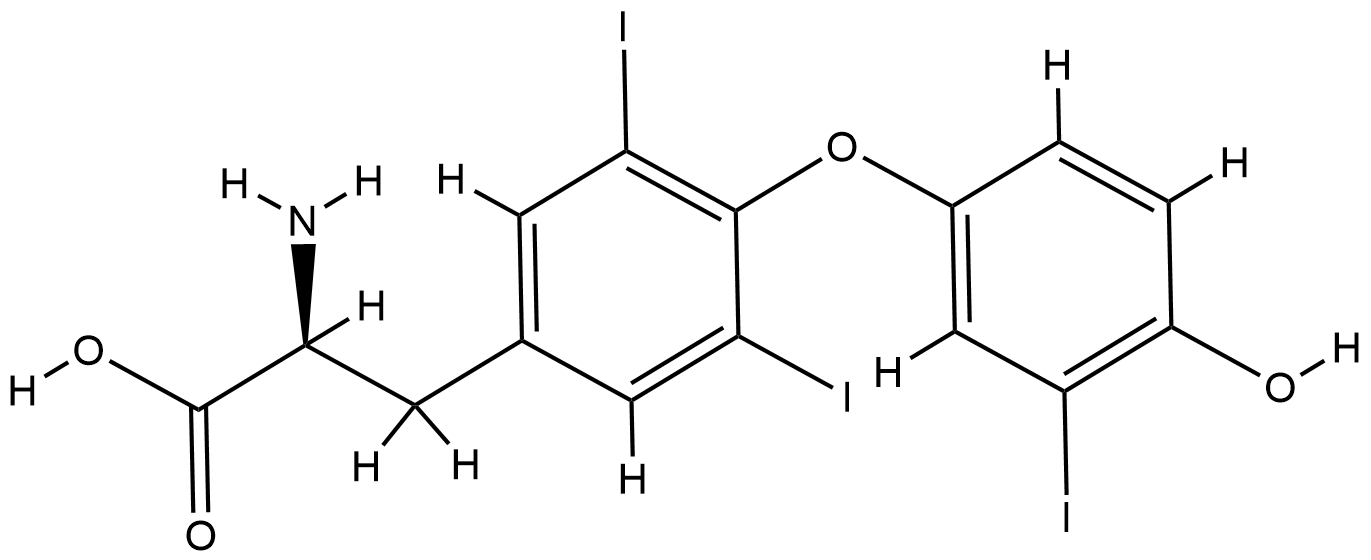
ClinicalTrials.gov (NCT04348513) Triiodothyronine for the Treatment of Critically Ill Patients With COVID-19 Infection. U.S. National Institutes of Health.
|
| 5 |
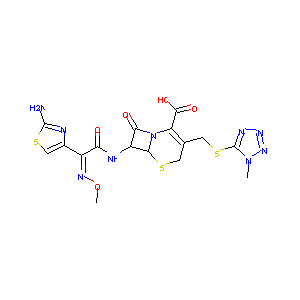
In vitro evaluation of E1040, a new cephalosporin with potent antipseudomonal activity. Antimicrob Agents Chemother. 1988 May;32(5):693-701.
|
|
|
|
|
|
|