| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
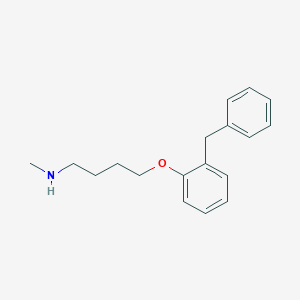
4-(O-benzylphenoxy)-N-methylbutylamine (bifemelane) and other 4-(O-benzylphenoxy)-N-methylalkylamines as new inhibitors of type A and B monoamine oxidase. J Neurochem. 1988 Jan;50(1):243-7.
|
| 3 |
The ChEMBL database in 2017. Nucleic Acids Res. 2017 Jan 4;45(D1):D945-D954.
|
| 4 |
Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27.
|
|
|
|
|
|
|