| 1 |
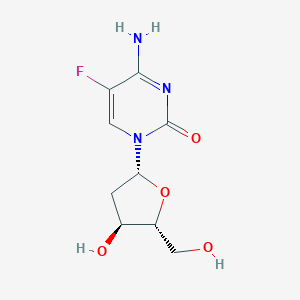
ClinicalTrials.gov (NCT01041443) 5-Fluoro-2'-Deoxycytidine and Tetrahydrouridine in Treating Patients With Acute Myeloid Leukemia or Myelodysplastic Syndromes
|
| 2 |
ClinicalTrials.gov (NCT00978250) A Multi-Histology Phase II Study of 5-Fluoro-2-Deoxycytidine With Tetrahydrouridine (FdCyd + THU). U.S. National Institutes of Health.
|
| 3 |
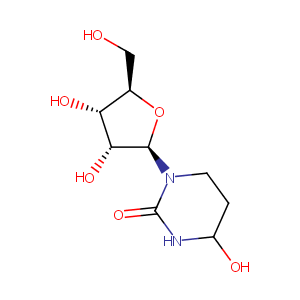
Quantitative determination of the cytidine deaminase inhibitor tetrahydrouridine (THU) in mouse plasma by liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2007;21(13):1991-7.
|
| 4 |
Tetrahydrouridine inhibits cell proliferation through cell cycle regulation regardless of cytidine deaminase expression levels. PLoS One. 2012;7(5):e37424.
|
| 5 |
Comparison of in vitro metabolic conversion of capecitabine to 5-FU in rats, mice, monkeys and humans--toxicological implications. J Toxicol Sci. 2011 Aug;36(4):411-22.
|
| 6 |
ClinicalTrials.gov (NCT00359606) 5-Fluoro-2'-Deoxcyctidine and Tetrahydrouridine to Treat Patients With Advanced Cancer
|
|
|
|
|
|
|