| 1 |
ClinicalTrials.gov (NCT02360592) Combination Therapy With Interferon Plus Interleukin 2 and Hepatitis B Vaccine in Chronic Hepatitis B Patients
|
| 2 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
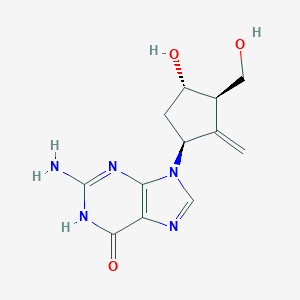
Effect of hepatitis B virus reverse transcriptase variations on entecavir treatment response. J Infect Dis. 2014 Sep 1;210(5):701-7.
|
| 5 |
Multiple SLC and ABC Transporters Contribute to the Placental Transfer of Entecavir. Drug Metab Dispos. 2017 Mar;45(3):269-278.
|
| 6 |
Human organic anion transporter 2 is an entecavir, but not tenofovir, transporter. Drug Metab Pharmacokinet. 2017 Feb;32(1):116-119.
|
| 7 |
The oligopeptide transporter 2-mediated reabsorption of entecavir in rat kidney. Eur J Pharm Sci. 2014 Feb 14;52:41-7.
|
| 8 |
6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon / by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol Appl Pharmacol. 2017 Dec 1;336:31-39. doi: 10.1016/j.taap.2017.10.004. Epub 2017 Oct 12.
|
| 9 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
|
|
|
|
|
|