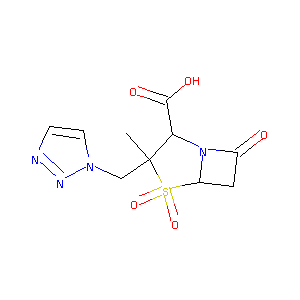
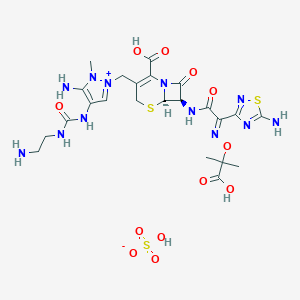
| 1 |
ClinicalTrials.gov (NCT03485950) Comparative Study To Determine The Efficacy, Safety, And Tolerability Of Ceftolozane-Tazobactam
|
| 2 |
Tazobactam FDA Label
|
| 3 |
Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007 Mar;70(3):461-77.
|
| 4 |
ClinicalTrials.gov (NCT02421120) Population Pharmacokinetics and Safety of Intravenous Ceftolozane/Tazobactam in Adult Cystic Fibrosis Patients. U.S. National Institutes of Health.
|
| 5 |
Selection of TNF-alpha binding affibody molecules using a beta-lactamase protein fragment complementation assay. N Biotechnol. 2009 Nov 30;26(5):251-9.
|
| 6 |
Characterization of piperacillin/tazobactam-resistant klebsiella oxytoca recovered from a nosocomial outbreak. PLoS One. 2015 Nov 5;10(11):e0142366.
|
| 7 |
Determination of the antibiotic resistance rates of Serratia marcescens isolates obtained from various clinical specimens. Niger J Clin Pract. 2019 Jan;22(1):125-130.
|
| 8 |
Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 2005 Aug;86(2):127-41.
|
|
|
|
|
|
|