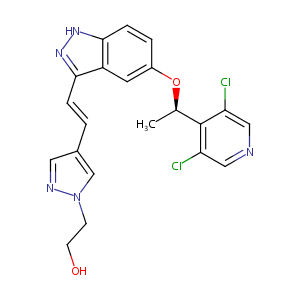
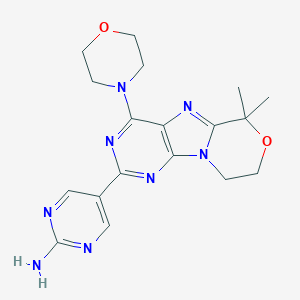
| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8104).
|
| 3 |
ClinicalTrials.gov (NCT03522298) Safety, Pharmacokinetics and Efficacy of Paxalisib (GDC-0084) in Newly-diagnosed Glioblastoma. U.S. National Institutes of Health.
|
| 4 |
Company report (Eli Lilly) (drug: LY2874455)
|
| 5 |
Current clinical development of PI3K pathway inhibitors in glioblastoma. Neuro Oncol. 2012 July; 14(7): 819-829.
|
|
|
|
|
|
|