Details of the Drug
General Information of Drug (ID: DMZ0DMY)
| Drug Name |
LY2874455
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
LY2874455; 1254473-64-7; LY-2874455; UNII-E9M363811V; CHEMBL3828009; E9M363811V; AK186860; LY 2874455; (R,E)-2-(4-(2-(5-(1-(3,5-Dichloropyridin-4-yl)-ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol; C21H19Cl2N5O2; (R,E)-2-(4-(2-(5-(1-(3,5-dichloropyridin-4-yl)ethoxy)-1H-indazol-3-yl)vinyl)-1H-pyrazol-1-yl)ethanol; GKJCVYLDJWTWQU-CXLRFSCWSA-N; SCHEMBL298446; SCHEMBL298445; QCR-90; GTPL8104; DTXSID20154776; MolPort-044-724-281; MolPort-023-219-117; EX-A1340; BCP04756; ZINC73069242; BDBM50189781; 2529AH; s7057; AKOS027251051
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
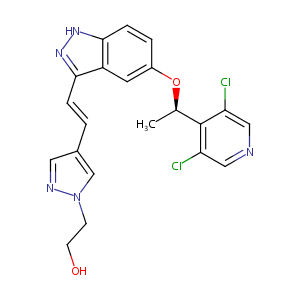 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 444.3 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 3.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Solid tumour/cancer | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | 2A00-2F9Z | |||||||||||||||||||||||
|
||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||
