| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Has nature already identified all useful antibacterial targets Curr Opin Microbiol. 2008 Oct;11(5):387-92.
|
| 3 |
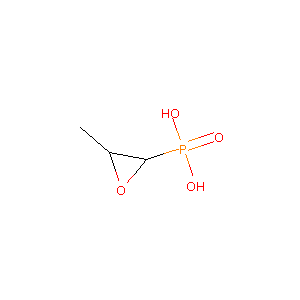
Fosfomycin FDA Label
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 5 |
Evidence that the fosfomycin target Cys115 in UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) is essential for product release. J Biol Chem. 2005 Feb 4;280(5):3757-63.
|
| 6 |
Formation of an adduct between fosfomycin and glutathione: a new mechanism of antibiotic resistance in bacteria. Antimicrob Agents Chemother. 1988 Oct;32(10):1552-6.
|
| 7 |
[Successful treatment of MRSA-associated glomerulonephritis with antibiotic therapy]. Nihon Jinzo Gakkai Shi. 2003;45(1):37-41.
|
| 8 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
|
|
|
|
|
|