| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
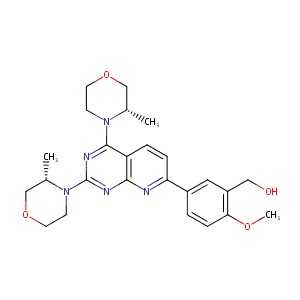
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7714).
|
| 3 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
ClinicalTrials.gov (NCT01106599) A Study Evaluating the Safety, Tolerability, and Pharmacokinetics of GDC-0623 in Patients With Locally Advanced or Metastatic Solid Tumors. U.S. National Institutes of Health.
|
| 6 |
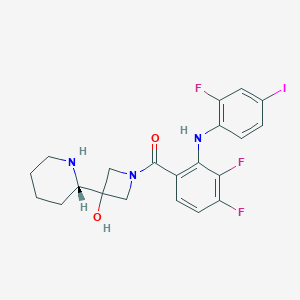
Clinical pipeline report, company report or official report of AstraZeneca (2009).
|
| 7 |
Frenolicin B Targets Peroxiredoxin 1 and Glutaredoxin 3 to Trigger ROS/4E-BP1-Mediated Antitumor Effects. Cell Chem Biol. 2019 Mar 21;26(3):366-377.e12. doi: 10.1016/j.chembiol.2018.11.013. Epub 2019 Jan 17.
|
|
|
|
|
|
|