| 1 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
| 2 |
ClinicalTrials.gov (NCT00089271) 17-DMAG in Treating Patients With Metastatic or Unresectable Solid Tumors or Lymphomas. U.S. National Institutes of Health.
|
| 3 |
ClinicalTrials.gov (NCT02049957) Safety and Efficacy Study of Sapanisertib in Combination With Exemestane or Fulvestrant in Postmenopausal Women With Estrogen Receptor Positive/Human Epidermal Growth Factor Receptor 2 Negative (ER+/HER2-) Metastatic Breast Cancer. U.S. National Institutes of Health.
|
| 4 |
Prevent COVID-19 Severity by Repurposing mTOR Inhibitors. 2 April 2020
|
| 5 |
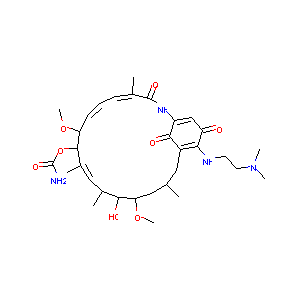
Stage 1 testing and pharmacodynamic evaluation of the HSP90 inhibitor alvespimycin (17-DMAG, KOS-1022) by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008 Jul;51(1):34-41.
|
| 6 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
|
|
|
|
|
|