| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7119).
|
| 3 |
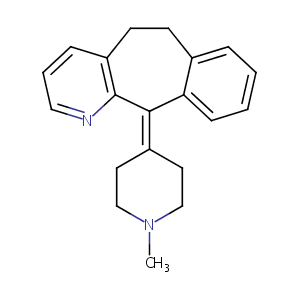
Azatadine FDA Label
|
| 4 |
Armodafinil FDA Label
|
| 5 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 6 |
Comparative effects of loratadine and azatadine in the treatment of seasonal allergic rhinitis. Asian Pac J Allergy Immunol. 1990 Dec;8(2):103-7.
|
| 7 |
Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 2007 June; 3(3): 349-364.
|
| 8 |
Interaction profile of armodafinil with medications metabolized by cytochrome P450 enzymes 1A2, 3A4 and 2C19 in healthy subjects. Clin Pharmacokinet. 2008;47(1):61-74.
|
| 9 |
Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet. 2003;42(2):123-37.
|
|
|
|
|
|
|