| 1 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 2 |
Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthritis Cartilage. 2003 Oct;11(10):738-46.
|
| 3 |
ClinicalTrials.gov (NCT03345095) A Phase III Trial of With Marizomib in Patients With Newly Diagnosed Glioblastoma (MIRAGE). U.S. National Institutes of Health.
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
Clinical pipeline report, company report or official report of Triphase Accelerator .
|
| 6 |
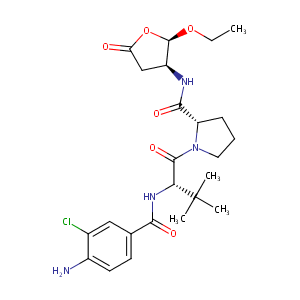
(S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydr... J Pharmacol Exp Ther. 2007 May;321(2):509-16.
|
| 7 |
Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011 Mar;11(3):254-84.
|
|
|
|
|
|
|