| 1 |
ClinicalTrials.gov (NCT05382312) Early Bactericidal Activity, Safety & Tolerability of Oral GSK3036656 in a Dual Combination With Novel and Established Antitubercular Agents, or Standard of Care in Adults With Rifampicin Susceptible Pulmonary Tuberculosis
|
| 2 |
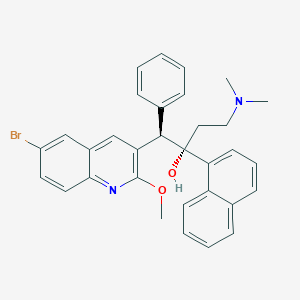
Bedaquiline FDA Label
|
| 3 |
Nat Rev Drug Discov. 2013 Feb;12(2):87-90.
|
| 4 |
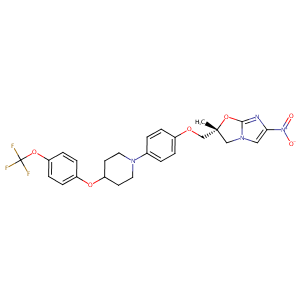
Compassionate use of delamanid in adults and children for drug-resistant tuberculosis: 5-year update. Eur Respir J. 2021 May 20;57(5):2002483.
|
| 5 |
ClinicalTrials.gov (NCT01424670) Safety and Efficacy Trial of Delamanid for 6 Months in Patients With Multidrug Resistant Tuberculosis. U.S. National Institutes of Health.
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
Bedaquiline metabolism: enzymes and novel metabolites. Drug Metab Dispos. 2014 May;42(5):863-6.
|
| 8 |
Pharmacokinetics and metabolism of delamanid, a novel anti-tuberculosis drug, in animals and humans: importance of albumin metabolism in vivo. Drug Metab Dispos. 2015 Aug;43(8):1267-76.
|
|
|
|
|
|
|