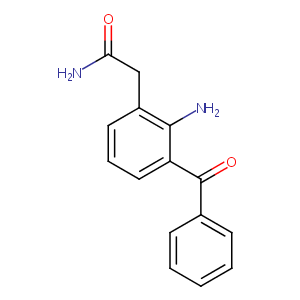
| 1 |
ClinicalTrials.gov (NCT01939691) Macular Edema Nepafenac vs. Difluprednate Uveitis Trial
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7474).
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7564).
|
| 4 |
2008 FDA drug approvals. Nat Rev Drug Discov. 2009 Feb;8(2):93-6.
|
| 5 |
Prevalence of non-cytochrome P450-mediated metabolism in food and drug administration-approved oral and intravenous drugs: 2006-2015. Drug Metab Dispos. 2016 Aug;44(8):1246-52.
|
| 6 |
Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003 Jan;44(1):409-15.
|
|
|
|
|
|
|