| 1 |
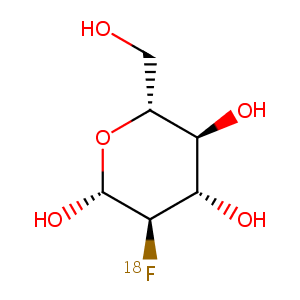
ClinicalTrials.gov (NCT02891616) 18F-FLT PET Imaging in Patients With Advanced Melanoma
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 3 |
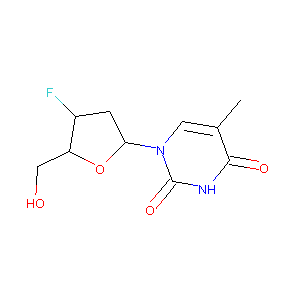
ClinicalTrials.gov (NCT02232581) Study to Determine the Antiviral Activity and Safety of Alovudine in Nucleoside-experienced HIV-infected Subjects Experiencing Virologic Failure. U.S. National Institutes of Health.
|
| 4 |
Multiple-dose pharmacokinetics of apricitabine, a novel nucleoside reverse transcriptase inhibitor, in patients with HIV-1 infection. Clin Drug Investig. 2008;28(2):129-38.
|
|
|
|
|
|
|