Details of the Drug
General Information of Drug (ID: DMJPV5X)
| Drug Name |
Alovudine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alovudine; 25526-93-6; FddT; 3'-DEOXY-3'-FLUOROTHYMIDINE; FddThD; 3'-Fluoro-3'-deoxythymidine; Thymidine, 3'-deoxy-3'-fluoro-; 3'F-TdR; 3'-FddT; Alovudine [USAN:INN]; UNII-PG53R0DWDQ; 3'-Fluorothymidine; 3'-FLT; DRG-0097; PG53R0DWDQ; NSC 140025; BRN 0754299; 3'-Fluorodeoxythymidine; CL 184824; CHEMBL105318; FLT; 1-((2R,4S,5R)-4-fluoro-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; C10H13FN2O4; MIV-310; 1-(3'-Deoxy-3'-fluoro-beta-D-pentofuranosyl)thymine; CL-184824; DSSTox_RID_81738; DSSTox_CID_26579
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
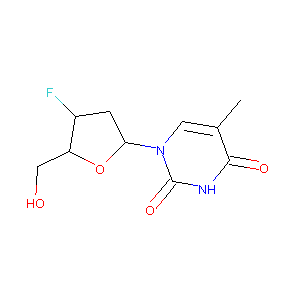 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 244.22 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 5 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
References
