| 1 |
ClinicalTrials.gov (NCT05686863) Remiazolam Combined With Esketamine in Painless Bidirectional Endoscopy in Children
|
| 2 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020
|
| 3 |
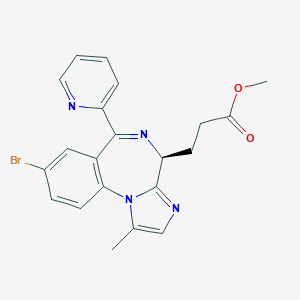
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8442).
|
| 4 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 5 |
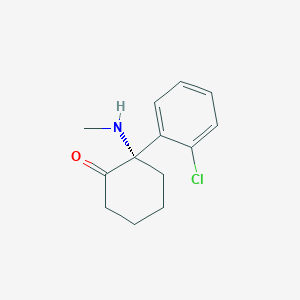
Contribution of CYP3A4, CYP2B6, and CYP2C9 isoforms to N-demethylation of ketamine in human liver microsomes. Drug Metab Dispos. 2002 Jul;30(7):853-8.
|
| 6 |
Metabolism and metabolomics of ketamine: a toxicological approach. Forensic Sci Res. 2017 Feb 20;2(1):2-10.
|
| 7 |
ClinicalTrials.gov (NCT05635955) Remimazolam Versus Propofol for Painless Abortion
|
|
|
|
|
|
|