Details of the Drug
General Information of Drug (ID: DMVU687)
| Drug Name |
Esketamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
(S)-Ketamine; l-Ketamine; (S)-(-)-Ketamine; 33643-46-8; S-Ketamine; UNII-50LFG02TXD; 50LFG02TXD; (S)-2-(o-chlorophenyl)-2-(methylamino)cyclohexanone; CHEBI:60799; esketamine HCl; (2S)-2-(2-chlorophenyl)-2-(methylamino)cyclohexanone; cyclohexanone, 2-(2-chlorophenyl)-2-(methylamino)-, (2S)-; 6740-88-1 (Parent); Esketamine [USAN:INN:BAN]; S-Ketamin; S-(-)-Ketamine; Esketamine (USAN/INN); AC1L4AD8; DSSTox_CID_27787; DSSTox_RID_82562; DSSTox_GSID_47810; SCHEMBL5512024; GTPL9152; CHEMBL395091; DTXSID6047810; YQEZ
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| ATC Code | |||||||||||||||||||||||
| Structure |
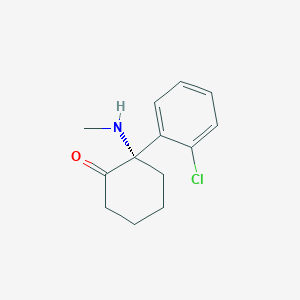 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 237.72 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.2 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Esketamine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||||||||
References
