| 1 |
Biologically active neutrophil chemokine pattern in tonsillitis.Clin Exp Immunol. 2004 Mar;135(3):511-8. doi: 10.1111/j.1365-2249.2003.02390.x.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 7330).
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
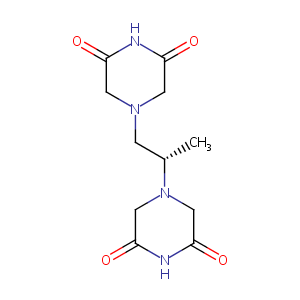
Dexrazoxane FDA Label
|
| 5 |
Design and development of antisense drugs. Expert Opin. Drug Discov. 2008 3(10):1189-1207.
|
| 6 |
How many drug targets are there Nat Rev Drug Discov. 2006 Dec;5(12):993-6.
|
| 7 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services.
|
| 8 |
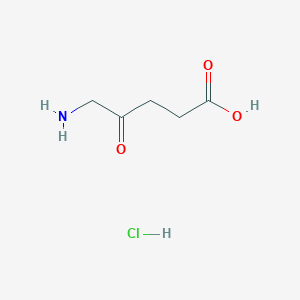
Metabolism of the cardioprotective drug dexrazoxane and one of its metabolites by isolated rat myocytes, hepatocytes, and blood. Drug Metab Dispos. 2005 Jun;33(6):719-25.
|
| 9 |
Investigation of novel dexrazoxane analogue JR-311 shows significant cardioprotective effects through topoisomerase IIbeta but not its iron chelating metabolite. Toxicology. 2017 Dec 1;392:1-10. doi: 10.1016/j.tox.2017.09.012. Epub 2017 Sep 21.
|
| 10 |
Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension.Hepatology. 2016 Jun;63(6):1977-86. doi: 10.1002/hep.28499. Epub 2016 Mar 31.
|
| 11 |
Loss of function mutations in VARS encoding cytoplasmic valyl-tRNA synthetase cause microcephaly, seizures, and progressive cerebral atrophy.Hum Genet. 2018 Apr;137(4):293-303. doi: 10.1007/s00439-018-1882-3. Epub 2018 Apr 24.
|
|
|
|
|
|
|