| 1 |
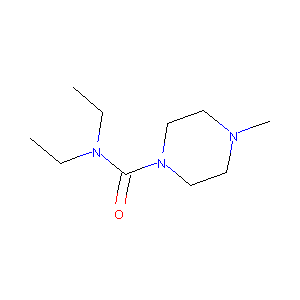
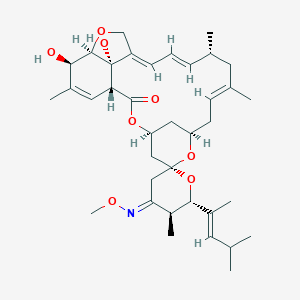
ClinicalTrials.gov (NCT04410406) Moxidectin for LF, Cote d'Ivoire (DOLF)
|
| 2 |
Diethylcarbamazine FDA Label
|
| 3 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 4 |
FDA Approved Drug Products from FDA Official Website. 2018. Application Number: (ANDA) 210867.
|
| 5 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 6 |
Inhibition of leukotriene formation by diethylcarbamazine modifies the acid-base balance in the rabbits with blast injuries of the lungs. Vojnosanit Pregl. 1999 May-Jun;56(3):243-7.
|
| 7 |
Application of higher throughput screening (HTS) inhibition assays to evaluate the interaction of antiparasitic drugs with cytochrome P450s. Drug Metab Dispos. 2001 Jan;29(1):30-5.
|
| 8 |
Reversal effects of two new milbemycin compounds on multidrug resistance in MCF-7/adr cells in vitro. Eur J Pharmacol. 2011 Jun 1;659(2-3):108-13. doi: 10.1016/j.ejphar.2011.03.023. Epub 2011 Mar 31.
|
| 9 |
In vitro and in vivo interaction of moxidectin with BCRP/ABCG2. Chem Biol Interact. 2009 Jun 15;180(1):106-12. doi: 10.1016/j.cbi.2009.02.009. Epub 2009 Feb 23.
|
|
|
|
|
|
|