Details of the Drug Combination
General Information of Drug Combination (ID: DCYGOD5)
| Drug Combination Name |
Itopride Norfloxacin
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indication |
|
|||||||||||||||||
| Component Drugs | Itopride | Norfloxacin | ||||||||||||||||
| N.A. | Small molecular drug | |||||||||||||||||

|
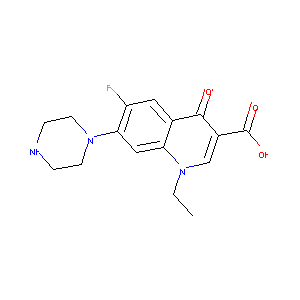
|
|||||||||||||||||
| 2D MOL | 2D MOL | |||||||||||||||||
| 3D MOL | 3D MOL | |||||||||||||||||
Molecular Interaction Atlas of This Drug Combination
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Itopride Interacts with 1 DME Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Itopride Interacts with 1 DOT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication(s) of Norfloxacin |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norfloxacin Interacts with 1 DTT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norfloxacin Interacts with 3 DTP Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norfloxacin Interacts with 1 DME Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Norfloxacin Interacts with 9 DOT Molecule(s)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
