Details of the Drug Combinations
General Information of This Drug (ID: DM0798Z)
| Drug Name | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
|
||||||||||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||||||||||
| Structure |
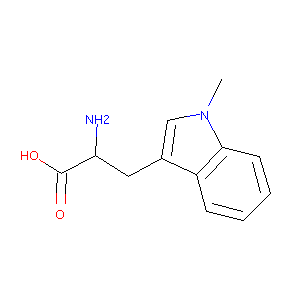 |
||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
5 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||
References
