Details of the Drug Combinations
General Information of This Drug (ID: DM0GUSX)
| Drug Name | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Achedos; Acidex; Alquen; Atural; Axoban; Biotidin; Coralen; Curan; Duractin; Ezopta; Fendibina; Gastrial; Gastridina; Gastrolav; Gastrosedol; Istomar; Kuracid; Logast; Mauran; Melfax; Microtid; Noctone; Ptinolin; Quantor; Quicran; RND; Radinat; Randin; Raniben; Raniberl; Ranibloc; Ranidine; Ranifur; Ranin; Raniogas; Raniplex; Ranisen; Raniter; Ranitidin; Ranitidina; Ranitidinum; Ranitiget; Ranitin; Rantacid; Rantidine; Ratic; Raticina; Sampep; Sostril; Taural; Terposen; Ulceranin; Ulcex; Ultidine; Urantac; Verlost; Vesyca; Vizerul; Weichilin; Weidos; Xanidine; ZANTAC; Zantab; Zantadin; Zantic; Ranitidine HCL; Rantidine HCL; Nu-Ranit; Rani-Q; Rani-nerton; Ranitidina [INN-Spanish]; Ranitidine (TN); Ranitidinum [INN-Latin]; Ul-Pep; Zantac (TN); Ranitidine (USAN/INN); Ranitidine [USAN:BAN:INN]; N-(2-((5-((Dimethylamino)methyl)furfuryl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; N (2-(((5-((Dimethylamino)methyl)-2-furanyl)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; (E)-1-N'-[2-[[5-(dimethylaminomethyl)furan-2-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({5-[(dimethylamino)methyl]furan-2-yl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitroethene-1,1-diamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiulcer Agents
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
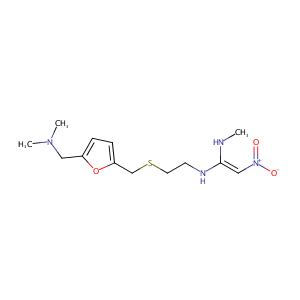 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
4 Clinical Trial Drug Combination(s) Consisting of This drug
|
||||||||||||||||||||||||||||||||||||||||
References
