Details of the Drug
General Information of Drug (ID: DM0GUSX)
| Drug Name |
Ranitidine
|
||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Achedos; Acidex; Alquen; Atural; Axoban; Biotidin; Coralen; Curan; Duractin; Ezopta; Fendibina; Gastrial; Gastridina; Gastrolav; Gastrosedol; Istomar; Kuracid; Logast; Mauran; Melfax; Microtid; Noctone; Ptinolin; Quantor; Quicran; RND; Radinat; Randin; Raniben; Raniberl; Ranibloc; Ranidine; Ranifur; Ranin; Raniogas; Raniplex; Ranisen; Raniter; Ranitidin; Ranitidina; Ranitidinum; Ranitiget; Ranitin; Rantacid; Rantidine; Ratic; Raticina; Sampep; Sostril; Taural; Terposen; Ulceranin; Ulcex; Ultidine; Urantac; Verlost; Vesyca; Vizerul; Weichilin; Weidos; Xanidine; ZANTAC; Zantab; Zantadin; Zantic; Ranitidine HCL; Rantidine HCL; Nu-Ranit; Rani-Q; Rani-nerton; Ranitidina [INN-Spanish]; Ranitidine (TN); Ranitidinum [INN-Latin]; Ul-Pep; Zantac (TN); Ranitidine (USAN/INN); Ranitidine [USAN:BAN:INN]; N-(2-((5-((Dimethylamino)methyl)furfuryl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; N (2-(((5-((Dimethylamino)methyl)-2-furanyl)methyl)thio)ethyl)-N'-methyl-2-nitro-1,1-ethenediamine; (E)-1-N'-[2-[[5-(dimethylaminomethyl)furan-2-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({5-[(dimethylamino)methyl]-2-furyl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitroethene-1,1-diamine; (E)-N-{2-[({5-[(dimethylamino)methyl]furan-2-yl}methyl)sulfanyl]ethyl}-N'-methyl-2-nitroethene-1,1-diamine
|
||||||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||||||
| Therapeutic Class |
Antiulcer Agents
|
||||||||||||||||||||||||||||||
| Affected Organisms |
Humans and other mammals
|
||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||||||
| Structure |
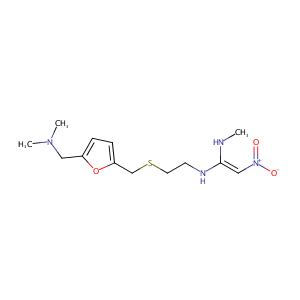 |
||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 314.41 | |||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 0.3 | ||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 9 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug-Metabolizing Enzyme (DME) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Ranitidine (Comorbidity)
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Ranitidine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 1234). | ||||
| 3 | Clinical pharmacokinetics of fingolimod. Clin Pharmacokinet. 2012 Jan 1;51(1):15-28. doi: 10.2165/11596550-000000000-00000. | ||||
| 4 | BDDCS predictions, self-correcting aspects of BDDCS assignments, BDDCS assignment corrections, and classification for more than 175 additional drugs | ||||
| 5 | FDA Approved Drug Products: ZANTAC (ranitidine hydrochloride) injection | ||||
| 6 | Clinical pharmacokinetics of ranitidine. Clin Pharmacokinet. 1984 May-Jun;9(3):211-21. doi: 10.2165/00003088-198409030-00003. | ||||
| 7 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 8 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 9 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 10 | Knockouts model the 100 best-selling drugs--will they model the next 100 Nat Rev Drug Discov. 2003 Jan;2(1):38-51. | ||||
| 11 | A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005 Oct;315(1):337-45. | ||||
| 12 | Differential substrate and inhibitory activities of ranitidine and famotidine toward human organic cation transporter 1 (hOCT1; SLC22A1), hOCT2 (SLC22A2), and hOCT3 (SLC22A3). J Pharmacol Exp Ther. 2005 Dec;315(3):1288-97. | ||||
| 13 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | ||||
| 14 | Oxidation of ranitidine by isozymes of flavin-containing monooxygenase and cytochrome P450. Jpn J Pharmacol. 2000 Oct;84(2):213-20. | ||||
| 15 | Comparative in vitro and in vivo inhibition of cytochrome P450 CYP1A2, CYP2D6, and CYP3A by H2-receptor antagonists. Clin Pharmacol Ther. 1999 Apr;65(4):369-76. | ||||
| 16 | Drug metabolism by flavin-containing monooxygenases of human and mouse. Expert Opin Drug Metab Toxicol. 2017 Feb;13(2):167-181. | ||||
| 17 | Effects of histamine H(2)-receptor antagonists on human plasma levels of calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J Pharm Pharmacol. 2002 Nov;54(11):1559-63. doi: 10.1211/002235702117. | ||||
| 18 | Evaluation of fresh and cryopreserved hepatocytes as in vitro drug metabolism tools for the prediction of metabolic clearance. Drug Metab Dispos. 2004 Nov;32(11):1247-53. doi: 10.1124/dmd.104.000026. Epub 2004 Jul 30. | ||||
| 19 | Effects of three H2-receptor antagonists (cimetidine, famotidine, ranitidine) on serum gastrin level. Int J Clin Pharmacol Res. 2002;22(2):29-35. | ||||
| 20 | [Impotence and gynecomastia secondary to hyperprolactinemia induced by ranitidine]. Therapie. 1994 Jul-Aug;49(4):361-2. | ||||
| 21 | Effects of histamine H2-receptor blockade on the cardiovascular reflex response to lower-body negative pressure in man. Acta Physiol Scand. 1990 May;139(1):161-72. doi: 10.1111/j.1748-1716.1990.tb08909.x. | ||||
| 22 | Albert KS, Welch RD, DeSante KA, DiSanto AR "Decreased tetracycline bioavailability caused by a bismuth subsalicylate antidiarrheal mixture." J Pharm Sci 68 (1979): 586-8. [PMID: 435335] | ||||
| 23 | Honig PK, Gillespie BK "Clinical significance of pharmacokinetic drug interactions with over-the-counter (OTC) drugs." Clin Pharmacokinet 35 (1998): 167-71. [PMID: 9784931] | ||||
| 24 | Dey NG, Castleden CM, Ward J, et al "The effect of cimetidine on tolbutamide kinetics." Br J Clin Pharmacol 16 (1983): 438-40. [PMID: 6626438] | ||||
| 25 | Beers MH, Ouslander JG, Rollingher I, Reuben DB, Brooks J, Beck JC "Explicit criteria for determining inappropriate medication use in nursing home residents." Arch Intern Med 151 (1991): 1825-32. [PMID: 1888249] | ||||
| 26 | Product Information. Tagamet (cimetidine). SmithKline Beecham, Philadelphia, PA. | ||||
| 27 | Alffenaar JW, van Assen S, van der Werf TS, Kosterink JG, Uges DR "Omeprazole significantly reduces posaconazole serum trough level." Clin Infect Dis 48 (2009): 839. [PMID: 19220151] | ||||
| 28 | Anderson JR, Poklis A, Slavin RG "A fatal case of theophylline intoxication." Arch Intern Med 143 (1983): 559-60. [PMID: 6830388] | ||||
| 29 | Product Information. Spectracef (cefditoren). TAP Pharmaceuticals Inc, Deerfield, IL. | ||||
| 30 | Cerner Multum, Inc. "Australian Product Information.". | ||||
| 31 | Product Information. Turalio (pexidartinib). Daiichi Sankyo, Inc., Parsippany, NJ. | ||||
| 32 | Cerner Multum, Inc. "UK Summary of Product Characteristics.". | ||||
| 33 | Katende RS, Dimich I "Resistance to nondepolarizing muscle relaxants in a patient treated with ranitidine." Mt Sinai J Med 54 (1987): 330-1. [PMID: 2955219] | ||||
| 34 | Blum RA, D'Andrea DT, Florentino BM, et al "Increased gastric pH and the bioavailability of fluconazole and ketoconazole." Ann Intern Med 114 (1991): 755-7. [PMID: 2012358] | ||||
| 35 | Adachi M, Hinatsu Y, et.al "Improved dissolution and absorption of ketoconazole in the presence of organic acids as pH-modifiers." Eur J Pharm Sci 76 (2015): 225-30. [PMID: 25988287] | ||||
| 36 | Product Information. Harvoni (ledipasvir-sofosbuvir). Gilead Sciences, Foster City, CA. | ||||
| 37 | Product Information. Lexiva (fosamprenavir). GlaxoSmithKline, Research Triangle Park, NC. | ||||
| 38 | Knupp CA, Graziano FM, Dixon RM, Barbhaiya RH "Pharmacokinetic-interaction study of didanosine and ranitidine in patients seropositive for human immunodeficiency virus." Antimicrob Agents Chemother 36 (1992): 2075-9. [PMID: 1444287] | ||||
| 39 | Product Information. Edurant (rilpivirine). Tibotec Pharmaceuticals, Titusville, NJ. | ||||
| 40 | Product Information. Isentress (raltegravir). Merck & Company Inc, West Point, PA. | ||||
| 41 | Product Information. Juxtapid (lomitapide). Aegerion Pharmaceuticals Inc, Cambridge, MA. | ||||
| 42 | Product Information. Zantac (ranitidine). Glaxo Wellcome, Research Triangle Park, NC. | ||||
| 43 | Product Information. Zykadia (ceritinib). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 44 | Product Information. Calquence (acalabrutinib). Astra-Zeneca Pharmaceuticals, Wilmington, DE. | ||||
| 45 | Product Information. Addyi (flibanserin). Sprout Pharmaceuticals, Raleigh, NC. | ||||
| 46 | Product Information. Gilenya (fingolimod). Novartis Pharmaceuticals, East Hanover, NJ. | ||||
| 47 | Product Information. Zeposia (ozanimod). Celgene Corporation, Summit, NJ. | ||||
| 48 | Product Information. Sprycel (dasatinib). Bristol-Myers Squibb, Princeton, NJ. | ||||
| 49 | Product Information. Effient (prasugrel). Lilly, Eli and Company, Indianapolis, IN. | ||||
| 50 | Product Information. Inlyta (axitinib). Pfizer U.S. Pharmaceuticals Group, New York, NY. | ||||
| 51 | Martin BK "Effect of ranitidine on procainamide disposition." Br J Clin Pharmacol 19 (1985): 858-60. [PMID: 4027133] | ||||
