Details of the Drug Combinations
General Information of This Drug (ID: DM0QZBN)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
BFQ; Acid ibandronico; Ibandronic Acid; Bisphosphonate 2; R484; RPR 102289A; Bondronat (TN); Boniva (TN); Bonviva (TN); Ibandronic acid (INN); Ibandronic acid [INN:BAN]; RPR-102289A; Ibandronic acid, sodium salt, monohydrate; Roche brand of ibandronic acid, sodium salt, monohydrate; [1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl]phosphonic acid; [1-HYDROXY-3-(METHYL-PENTYL-AMINO)-1-PHOSPHONO-PROPYL]-PHOSPHONIC ACID; (1-Hydroxy-3-(methylpentylamino)propylidene)diphosphonic acid; (1-hydroxy-3-(methylpentylamino)propylidene)bisphosphonate; 1-hydroxy-3-(methylpentylamino)propylidenebisphosphonate
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Bone Density Conservation Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
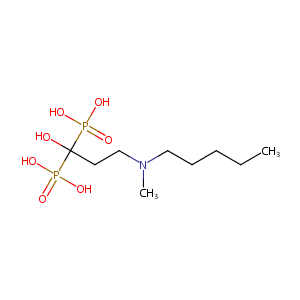 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
1 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||
References
