Details of the Drug Combinations
General Information of This Drug (ID: DM13VTW)
| Drug Name | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alcobon; Ancobon; Ancotil; Ancotyl; Flucitosina; Flucystine; Flucytosin; Flucytosinum; Flucytosone; Fluocytosine; Fluorcytosine; Fluorocytosine; Flucitosina [DCIT]; F0321; LT00771985; Ancobon (TN); Flucytosinum [INN-Latin]; GL663142 & 5FC; Ro 2-9915; Ro 29915 E/265601; Ro-2-9915; Flucytosine (JP15/USP/INN); Flucytosine [USAN:INN:BAN:JAN]; Cytosine, 5-fluoro-(6CI,7CI,8CI); GL663142 & 4-Amino-5-fluoropyrimidin-2(1H)-one; 2(1H)-Pyrimidinone, 4-amino-5-fluoro-); 2-Hydroxy-4-amino-5-fluoropyrimidine; 4-Amino-5-fluoro-2(1H)-pyrimidinone; 4-Amino-5-fluoro-2-hydroxypyrimidine; 4-Amino-5-fluoro-2-hyroxypyrimidine; 4-Amino-5-fluoropyrimidin-2(1H)-one; 5-FC; 5-Flucytosine; 5-Fluorocystosine; 5-Fluorocytosin; 5-Fluorocytosine; 5-Fluorocytosine-6-3H; 5-Flurocytosine; 5-fluoro cytosine; 5987P; 6-Amino-2-oxo-5-fluoropyrimidine; 6-amino-5-fluoro-1H-pyrimidin-2-one; 9074P
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
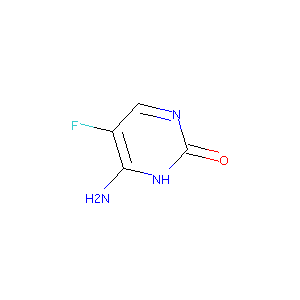 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Investigative Drug Combination(s) Consisting of This drug
Normalized Drug Combination Synergy Score
Synergy scores were normalized using Min-Max Scaling to facilitate visual comparisons.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
2 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
