Details of the Drug
General Information of Drug (ID: DM13VTW)
| Drug Name |
Flucytosine
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Alcobon; Ancobon; Ancotil; Ancotyl; Flucitosina; Flucystine; Flucytosin; Flucytosinum; Flucytosone; Fluocytosine; Fluorcytosine; Fluorocytosine; Flucitosina [DCIT]; F0321; LT00771985; Ancobon (TN); Flucytosinum [INN-Latin]; GL663142 & 5FC; Ro 2-9915; Ro 29915 E/265601; Ro-2-9915; Flucytosine (JP15/USP/INN); Flucytosine [USAN:INN:BAN:JAN]; Cytosine, 5-fluoro-(6CI,7CI,8CI); GL663142 & 4-Amino-5-fluoropyrimidin-2(1H)-one; 2(1H)-Pyrimidinone, 4-amino-5-fluoro-); 2-Hydroxy-4-amino-5-fluoropyrimidine; 4-Amino-5-fluoro-2(1H)-pyrimidinone; 4-Amino-5-fluoro-2-hydroxypyrimidine; 4-Amino-5-fluoro-2-hyroxypyrimidine; 4-Amino-5-fluoropyrimidin-2(1H)-one; 5-FC; 5-Flucytosine; 5-Fluorocystosine; 5-Fluorocytosin; 5-Fluorocytosine; 5-Fluorocytosine-6-3H; 5-Flurocytosine; 5-fluoro cytosine; 5987P; 6-Amino-2-oxo-5-fluoropyrimidine; 6-amino-5-fluoro-1H-pyrimidin-2-one; 9074P
|
||||||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||||||
| Therapeutic Class |
Antifungal Agents
|
||||||||||||||||||||||||||
| Affected Organisms |
Yeast and other fungi
|
||||||||||||||||||||||||||
| ATC Code |
|
||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||||||
| Structure |
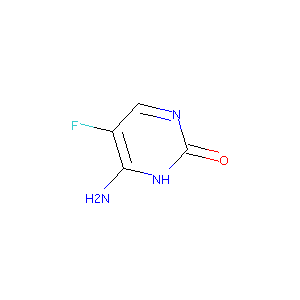 |
||||||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 129.09 | |||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -0.9 | ||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 0 | ||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 3 | ||||||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||||||
| Adverse Drug Reaction (ADR) |
|
||||||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | ||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Drug-Drug Interaction (DDI) Information of This Drug
|
Coadministration of a Drug Treating the Disease Different from Flucytosine (Comorbidity)
|
|||||||||||||||||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
References
| 1 | Flucytosine FDA Label | ||||
|---|---|---|---|---|---|
| 2 | Antifungal agents: mode of action in yeast cells. Rev Esp Quimioter. 2006 Jun;19(2):130-9. | ||||
| 3 | BDDCS applied to over 900 drugs | ||||
| 4 | Critical Evaluation of Human Oral Bioavailability for Pharmaceutical Drugs by Using Various Cheminformatics Approaches | ||||
| 5 | Trend Analysis of a Database of Intravenous Pharmacokinetic Parameters in Humans for 1352 Drug Compounds | ||||
| 6 | Estimating the safe starting dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose | ||||
| 7 | ADReCS-Target: target profiles for aiding drug safety research and application. Nucleic Acids Res. 2018 Jan 4;46(D1):D911-D917. doi: 10.1093/nar/gkx899. | ||||
| 8 | Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/ 5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005 Feb 10;24(7):1231-43. doi: 10.1038/sj.onc.1208290. | ||||
| 9 | Product Information. Ocrevus (ocrelizumab). Genentech, South San Francisco, CA. | ||||
| 10 | Product Information. Synribo (omacetaxine). Teva Pharmaceuticals USA, North Wales, PA. | ||||
| 11 | Cerner Multum, Inc. "Australian Product Information.". | ||||
