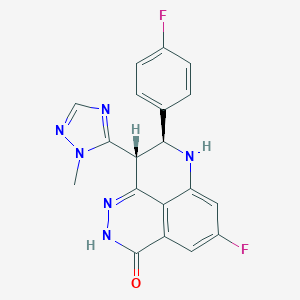
| 1 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 2 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8313).
|
| 3 |
ClinicalTrials.gov (NCT03875313) Study of CB-839 (Telaglenastat) in Combination With Talazoparib in Patients With Solid Tumors
|
| 4 |
ClinicalTrials.gov (NCT05898399) Study of ART6043 in Advanced/Metastatic Solid Tumors Patients
|
| 5 |
ClinicalTrials.gov (NCT05101551) Study of Talazoparib in Combination With Chemotherapy in Relapsed Pediatric AML to Determine Safety and Efficacy
|
| 6 |
ClinicalTrials.gov (NCT04703920) Talazoparib in Combination With Belinostat for Metastatic Breast Cancer, Metastatic Castration Resistant Prostate Cancer, and Metastatic Ovarian Cancer
|
| 7 |
ClinicalTrials.gov (NCT05035745) Selinexor & Talazoparib in Advanced Refractory Solid Tumors; Advanced/Metastatic Triple Negative Breast Cancer (START)
|
| 8 |
ClinicalTrials.gov (NCT04846478) Phase Ia/Ib Talazoparib + Tazemetostat for mCRPC
|
| 9 |
ClinicalTrials.gov (NCT03077607) A Study to Evaluate the Effect of Itraconazole and Rifampin on the Pharmacokinetics of Talazoparib in Patients With Advanced Solid Tumors
|
| 10 |
ClinicalTrials.gov (NCT03974217) Talazoparib for Cohesin-Mutated AML and MDS With Excess Blasts
|
| 11 |
ClinicalTrials.gov (NCT04337970) Talazoparib and Axitinib for People With Previously Treated Advanced Kidney Cancer
|
| 12 |
ClinicalTrials.gov (NCT06218628) Pacritinib w/ Talazoparib in Pts w/ Myeloproliferative Neoplasms Unresponsive to JAK2 Inhibition
|
| 13 |
ClinicalTrials.gov (NCT03637491) A Study of Avelumab, Binimetinib and Talazoparib in Patients With Locally Advanced or Metastatic RAS-mutant Solid Tumors
|
| 14 |
ClinicalTrials.gov (NCT02878785) Decitabine and Talazoparib in Untreated AML and R/R AML
|
| 15 |
ClinicalTrials.gov (NCT03911973) Gedatolisib Plus Talazoparib in Advanced Triple Negative or BRCA1/2 Positive, HER2 Negative Breast Cancers
|
| 16 |
ClinicalTrials.gov (NCT04332744) Enzalutamide Plus Talazoparib for the Treatment of Hormone Sensitive Prostate Cancer (ZZ-First)
|
| 17 |
ClinicalTrials.gov (NCT02836028) A Study Evaluating Talazoparib in Relapsed Ovarian, Fallopian Tube, and Peritoneal Cancer
|
|
|
|
|
|
|