| 1 |
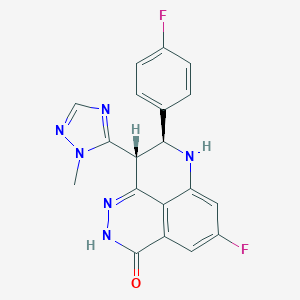
ClinicalTrials.gov (NCT04846478) Phase Ia/Ib Talazoparib + Tazemetostat for mCRPC
|
| 2 |
2018 FDA drug approvals.Nat Rev Drug Discov. 2019 Feb;18(2):85-89.
|
| 3 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 8313).
|
| 4 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health Human Services. 2020
|
| 5 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 6 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 7 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 8 |
DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB11760)
|
| 9 |
BMN 673 (talazoparib): A potent PARP inhibitor for triple negative breast cancer with different genetic profile. J Biochem Mol Toxicol. 2019 May;33(5):e22286. doi: 10.1002/jbt.22286. Epub 2019 Jan 23.
|
| 10 |
Structural Basis for Potency and Promiscuity in Poly(ADP-ribose) Polymerase (PARP) and Tankyrase Inhibitors. J Med Chem. 2017 Feb 23;60(4):1262-1271. doi: 10.1021/acs.jmedchem.6b00990. Epub 2016 Dec 21.
|
| 11 |
Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet. 2018 Aug;50(8):1086-1092. doi: 10.1038/s41588-018-0170-4. Epub 2018 Jul 16.
|
|
|
|
|
|
|