Details of the Drug Combinations
General Information of This Drug (ID: DM1N62C)
| Drug Name | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
quetiapine; 111974-69-7; Quetiapine [INN:BAN]; UNII-BGL0JSY5SI; BGL0JSY5SI; CHEMBL716; 2-[2-(4-Dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]ethanol; CHEBI:8707; C21H25N3O2S; URKOMYMAXPYINW-UHFFFAOYSA-N; 2-(2-(4-Dibenzo(b,f)(1,4)thiazepin-11-yl-1-piperazinyl)ethoxy)ethanol; Ethanol, 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-yl-1-piperazinyl)ethoxy]-; NCGC00095911-03; Co-Quetiapine; Norsic; Quetiapina; Quetiapinum; Quetiapine hemifumarate; Ketipinor (TN); Norsic (TN); Quetiapine (INN); Seroquel (Fumarate); Seroquel (TN); 2-[2-(4-dibenzo[b,f][1,4]thiazepin-11-ylpiperazin-1-yl)ethoxy]ethanol; 2-{[2-(4-dibenzo[b,f][1,4]thiazepin-11-ylpiperazin-1-yl)ethyl]oxy}ethanol; PD-172760
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antipsychotic Agents
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
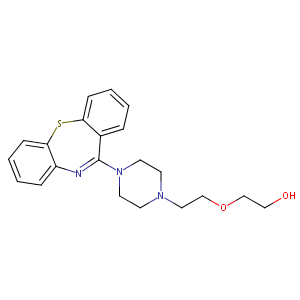 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of Combinatorial Drugs (CBD) Containing This Drug
|
6 Clinical Trial Drug Combination(s) Consisting of This drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
