| 1 |
ClinicalTrials.gov (NCT01129674) A Long-Term, Open-Label, Study on Schizophrenia
|
| 2 |
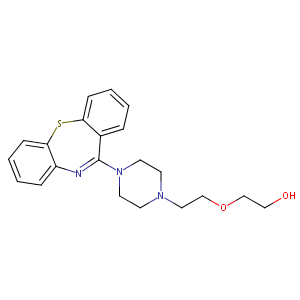
Quetiapine FDA Label
|
| 3 |
The pipeline and future of drug development in schizophrenia. Mol Psychiatry. 2007 Oct;12(10):904-22.
|
| 4 |
ClinicalTrials.gov (NCT02362412) Study to Evaluate the Effects of Switching Different Strength Forms of FK949E in Bipolar Disorder Patients With Major Depressive Episodes. U.S. National Institutes ofHealth.
|
| 5 |
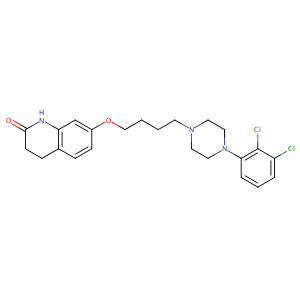
Aripiprazole FDA Label
|
| 6 |
Drugs@FDA. U.S. Food and Drug Administration. U.S. Department of Health & Human Services. 2015
|
| 7 |
URL: http://www.guidetopharmacology.org Nucleic Acids Res. 2015 Oct 12. pii: gkv1037. The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. (Ligand id: 34).
|
| 8 |
Trusted, scientifically sound profiles of drug programs, clinical trials, safety reports, and company deals, written by scientists. Springer. 2015. Adis Insight (drug id 800034267)
|
| 9 |
Clinical pipeline report, company report or official report of the Pharmaceutical Research and Manufacturers of America (PhRMA)
|
| 10 |
Receptor reserve-dependent properties of antipsychotics at human dopamine D2 receptors. Eur J Pharmacol. 2009 Apr 1;607(1-3):35-40.
|
| 11 |
Identification of P-glycoprotein substrates and inhibitors among psychoactive compounds--implications for pharmacokinetics of selected substrates. J Pharm Pharmacol. 2004 Aug;56(8):967-75.
|
| 12 |
Genome-wide association study of antipsychotic-induced QTc interval prolongation. Pharmacogenomics J. 2012 Apr;12(2):165-72.
|
| 13 |
Metabolism of the active metabolite of quetiapine, N-desalkylquetiapine in vitro. Drug Metab Dispos. 2012 Sep;40(9):1778-84.
|
| 14 |
A liquid chromatographic-electrospray-tandem mass spectrometric method for quantitation of quetiapine in human plasma and liver microsomes: application to study in vitro metabolism. J Anal Toxicol. 2004 Sep;28(6):443-8.
|
| 15 |
Influence of ABCB1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of quetiapine in healthy volunteers. Pharmacogenet Genomics. 2014 Jan;24(1):35-42.
|
| 16 |
Aripiprazole acts as a selective dopamine D2 receptor partial agonist. Expert Opin Investig Drugs. 2007 Jun;16(6):771-5.
|
| 17 |
Aripiprazole: a review of its use in schizophrenia and schizoaffective disorder. Drugs. 2004;64(15):1715-36.
|
| 18 |
Drug Interactions Flockhart Table
|
| 19 |
Small Molecule Antipsychotic Aripiprazole Potentiates Ozone-Induced Inflammation in Airway Epithelium. Chem Res Toxicol. 2019 Oct 21;32(10):1997-2005. doi: 10.1021/acs.chemrestox.9b00149. Epub 2019 Sep 11.
|
| 20 |
The efficacy, safety, and tolerability of aripiprazole for the treatment of schizoaffective disorder: results from a pooled analysis of a sub-population of subjects from two randomized, double-blind, placebo-controlled, pivotal trials. J Affect Disord. 2009 May;115(1-2):18-26. doi: 10.1016/j.jad.2008.12.017. Epub 2009 Feb 23.
|
| 21 |
Design and synthesis of novel arylpiperazine derivatives containing the imidazole core targeting 5-HT(2A) receptor and 5-HT transporter. J Med Chem. 2011 Sep 22;54(18):6305-18. doi: 10.1021/jm200682b. Epub 2011 Aug 23.
|
| 22 |
Why are most phospholipidosis inducers also hERG blockers?. Arch Toxicol. 2017 Dec;91(12):3885-3895. doi: 10.1007/s00204-017-1995-9. Epub 2017 May 27.
|
| 23 |
Pharmacologic analysis of non-synonymous coding h5-HT2A SNPs reveals alterations in atypical antipsychotic and agonist efficacies. Pharmacogenomics J. 2006 Jan-Feb;6(1):42-51. doi: 10.1038/sj.tpj.6500342.
|
| 24 |
ClinicalTrials.gov (NCT01164059) Clinical Effectiveness of Newer Antipsychotics in Comparison With Conventional Antipsychotics in Schizophrenia
|
|
|
|
|
|
|